AstraZeneca: FDA Accepts Imfinzi Supplemental Biologics License Application; Grants Priority Review
17 Outubro 2017 - 5:01AM
Dow Jones News
By Josee Rose
AstraZeneca PLC (AZN) on Tuesday said the U.S. Food and Drug
Administration has accepted a supplemental biologics license
application for Imfinzi, granting the drug Priority Review
status.
Imfinzi is being developed to treat patients with stage 3
unresectable non-small cell lung cancer whose disease hasn't
progressed after some chemoradiation therapy.
Imfinzi has had positive data from a phase 3 trial, which
continues to evaluate overall survival, one of its primary
endpoints.
Write to Josee Rose at josee.rose@wsj.com.
(END) Dow Jones Newswires
October 17, 2017 02:46 ET (06:46 GMT)
Copyright (c) 2017 Dow Jones & Company, Inc.
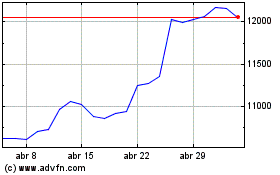
Astrazeneca (LSE:AZN)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
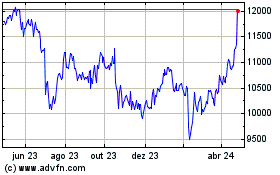
Astrazeneca (LSE:AZN)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024