MedinCell: US Pharma Development Expert Richard Malamut, MD Becomes Chief Medical Officer
09 Maio 2022 - 2:06PM
Business Wire
- Dr. Rick Malamut is an expert in regulatory processes and drug
development
- He oversaw the early clinical strategy of mdc-IRM at Teva
(2013-2016)
- He is a former member of MedinCell’s Medical Advisory Board and
MedinCell’s board of directors’ observer
- He joins MedinCell as part of the newly created US
subsidiary
"After working as an active member of the Medical Advisory Board
of MedinCell, I am delighted to be joining full time the company as
a CMO at a time when its first product is in the final stage of the
FDA approval process. I look forward to contribute to the success
of the other programs in MedinCell’s pipeline. MedinCell’s
breakthrough technology allows for significant clinical
improvements to existing medications and will provide meaningful
benefits to patients across a broad range of therapeutic
indications," said Dr Malamut.
“I am truly delighted. We’ve known Richard since 2013 when he
was at Teva. Richard is going to bring MedinCell his deep knowledge
of U.S. regulatory processes and clinical development, as well as
be instrumental in evaluating new opportunities. He is an expert in
both central nervous system and pain, where we are very active,"
said Christophe Douat, CEO of MedinCell. His input will be major
for the next stage of the company as more and more of our programs
have reached regulatory stage, and more to come, with most of
clinical development activities conducted in the U.S.”
Click here to access the complete press release
View source
version on businesswire.com: https://www.businesswire.com/news/home/20220509006001/en/
MedinCell David Heuzé Head of communication
david.heuze@medincell.com +33 (0)6 83 25 21 86
NewCap Louis-Victor Delouvrier / Olivier Bricaud Investor
Relations medincell@newcap.eu +33 (0)1 44 71 94 94
NewCap Nicolas Merigeau Media Relations medincell@newcap.eu +33
(0)1 44 71 94 94
Medincell (EU:MEDCL)
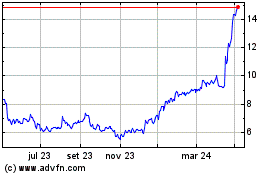
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
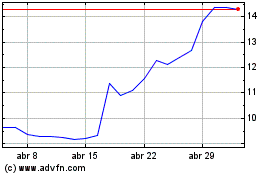
Medincell (EU:MEDCL)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024