- Study shows Velo consumers had significantly better results for
several indicators linked to smoking-related diseases compared with
smokers
- Results provide important new data and insights into the
real-world health impact that underscores the Tobacco Harm
Reduction potential of oral nicotine pouches
- Study adds to the weight of evidence that supports Velo as a
reduced-risk* product for smokers who completely switch from
smoking
- Results reinforce BAT’s ability to deliver A Better TomorrowTM
by reducing the health impact of its business
New results from an innovative cross-sectional clinical study of
Velo i, BAT’s flagship modern oral nicotine pouch product, have
been published today in Biomarkers Journal. In the study, consumers
exclusively using Velo for over six months had significant
favourable differences in several biomarkers of exposure and
biomarkers of potential harm relevant to smoking-related diseases
compared to the adults who smoked.
This press release features multimedia. View
the full release here:
https://www.businesswire.com/news/home/20221205005383/en/
A Velo modern oral product tested in BAT
laboratory, Southampton UK (Photo: Business Wire)
The results showed that the levels for the biomarkers of
exposure, based on priority toxicants as defined by the WHO ii,
were substantially lower in Velo consumers compared with smokers.
The data also showed favourable differences between the Velo
consumers and smokers in the majority of the biomarkers of
potential harm, with four achieving statistical significance, and
the others having similar levels across the Velo consumers, former
and never smoker groups.
The study provides important new data and insights into the
real-world health impact in Velo consumers compared to smokers,
former smokers and never smokers. A single set of samples of blood,
urine and other clinical measurements was tested for certain
toxicants and a range of biomarkers thought to be linked to the
development of diseases such as cancer and cardiovascular disease
(CVD).
Dr David O’Reilly, Director, Scientific Research at BAT,
said: “These results are very important for Velo and the modern
oral nicotine product category. They build on the extensive
scientific evidence, including epidemiological data, that already
exists for oral tobacco and add to the weight of evidence that
supports our belief that Velo is a reduced-risk*† product for
smokers who completely switch from cigarettes as compared to
continued smoking. We have already generated data that shows Velo
has a toxicant profile better than snus and comparable to Nicotine
Replacement Therapy (NRT)iii. These results add further evidence
that supports the important contribution Velo can make to Tobacco
Harm Reduction.
“I’d like to thank everyone who participated and helped deliver
this study. It is another important step forward in our journey to
building A Better Tomorrow.”
Based on the biomarkers measured, compared to smokers, Velo
consumers who had been using the product exclusively
showed:
- Significantly lower levels in biomarkers of exposure to
priority tobacco toxicants
- Significant favourable differences in a biomarker of potential
harm relevant to lung cancer risk
- Significant favourable differences in a number of biomarkers of
potential harm relevant to cardiovascular disease
- Significant favourable differences in a biomarker of potential
harm relevant to general inflammation
For the biomarkers that showed no significant difference between
the Velo consumers and smokers, similar levels were observed
between the Velo and former and never smoker groups.
About the study
The study included participants who had been using Velo
exclusively for over six months, as well as current smokers, former
smokers and never smokers. For the Velo consumers and current
cigarette smokers, usage patterns and overall consumption were not
controlled under the study protocol as the aim was to assess the
impact among people using the products in their ‘normal’ way rather
than in a controlled way. Four different groups were enrolled and
studied.
These comprised:
- Current smokers who had been smoking for at least one year
prior to screening
- Exclusive Velo consumers for at least six months
- Former smokers who had quit for at least six months
- Never smokers
Participants were based in Denmark and Sweden, aged between
19-55 years old, and in good general health.
Unlike longitudinal studies where participants attend multiple
clinic visits over an extended period of time, participants in this
study made a single clinic visit where samples of blood, urine and
breath were collected, and other clinical measurements were
performed. These samples and measurements were then assessed for
biomarkers of exposure (to selected toxicants) and biomarkers of
potential harm. Differences in the biomarker levels between the
groups were compared and analysed.
In addition, to ensure compliance with reported product usage,
the Velo and former smoker groups were tested for the biomarker,
CEVal, to indicate whether or not they had smoked cigarettes during
the preceding six months.
ENDS
About BAT
BAT is a leading, multi-category consumer goods business with a
purpose to build A Better Tomorrow™ by reducing the health impact
of its business through offering a greater choice of enjoyable and
less risky products for adult consumers.
The company continues to be clear that combustible cigarettes
pose serious health risks, and the only way to avoid these risks is
not to start or to quit. BAT encourages those who would otherwise
continue to smoke to switch completely to
scientifically-substantiated, reduced-risk alternatives*†. In order
to deliver this, BAT is transforming into a truly consumer-centric
multi-category consumer products business.
BAT’s ambition is to have 50 million consumers of its
non-combustible products by 2030 and to generate £5billion of New
Categories revenue by 2025. BAT has set stretching ESG targets
including achieving carbon neutrality for Scopes 1 & 2 by 2030
and eliminating unnecessary single-use plastic and making all
plastic packaging reusable, recyclable or compostable by 2025.
BAT employs over 52,000 people and operates in over 175
countries. The BAT Group generated revenue of £25.68 billion in
2021 and profit from operations of £10.2 billion.
The company’s Strategic Portfolio is made up of its global
cigarette brands and a growing range of reduced-risk*† New Category
tobacco and nicotine products and traditional non-combustible
tobacco products. These include vapour, tobacco heating products,
modern oral products including tobacco-free nicotine pouches, as
well as traditional oral products such as snus and moist snuff. In
the first half of 2022, we had 20.4 million consumers of our
non-combustible products, a rise of 2.1 million on full year
2021.
* Based on the weight of evidence and assuming a complete switch
from cigarette smoking. These products are not risk free and are
addictive.
† Our products as sold in the US, including Vuse, Velo, Grizzly,
Kodiak, and Camel Snus, are subject to Food and Drug Administration
(FDA) regulation and no reduced-risk claims will be made as to
these products without agency clearance.
This press release is not intended as a piece of promotional
material for any products, very notably in the US where claims of a
certain type are subject to FDA clearance. This update relates to
new scientific data and is not aimed at a specific market. It is
intended to provide further scientific evidence to underpin our
products.
Forward-looking statements
This release contains certain forward-looking statements,
including "forward-looking" statements made within the meaning of
the U.S. Private Securities Litigation Reform Act of 1995. These
statements are often, but not always, made through the use of words
or phrases such as "believe," "anticipate," "could," "may,"
"would," "should," "intend," "plan," "potential," "predict,"
"will," "expect," "estimate," "project," "positioned," "strategy,"
"outlook", "target" and similar expressions. These include
statements regarding our customer target ambition, New Categories
revenue targets and our ESG targets.
All such forward-looking statements involve estimates and
assumptions that are subject to risks, uncertainties and other
factors. It is believed that the expectations reflected in this
release are reasonable but they may be affected by a wide range of
variables that could cause actual results to differ materially from
those currently anticipated. A review of the reasons why actual
results and developments may differ materially from the
expectations disclosed or implied within forward-looking statements
can be found by referring to the information contained under the
headings “Cautionary Statement” and "Group Principal Risks " in the
2021 Annual Report and Form 20-F of British American Tobacco p.l.c.
(BAT).
Additional information concerning these and other factors can be
found in BAT's filings with the U.S. Securities and Exchange
Commission ("SEC"), including the Annual Report on Form 20-F and
Current Reports on Form 6-K, which may be obtained free of charge
at the SEC's website, http://www.sec.gov and BAT’s Annual Reports,
which may be obtained free of charge from the BAT website
www.bat.com.
Past performance is no guide to future performance and persons
needing advice should consult an independent financial adviser. The
forward-looking statements reflect knowledge and information
available at the date of preparation of this release and BAT
undertakes no obligation to update or revise these forward-looking
statements, whether as a result of new information, future events
or otherwise. Readers are cautioned not to place undue reliance on
such forward-looking statements.
i The study was conducted using Lyft, since re-branded as
Velo.
ii WHO Study Group on Tobacco Regulation. Report on the
Scientific Basis of Tobacco Product Regulation: Fifth Report of a
WHO Study Group. 2015.
iii David Azzopardi, Chuan Liu & James Murphy (2021)
Chemical characterization of tobacco-free “modern” oral nicotine
pouches and their position on the toxicant and risk continuums,
Drug and Chemical Toxicology, DOI:
10.1080/01480545.2021.1925691
View source
version on businesswire.com: https://www.businesswire.com/news/home/20221205005383/en/
Enquiries
Media Centre +44 (0) 20 7845 2888 (24
hours) | @BATplc
Investor Relations Victoria Buxton: +44 (0)20 7845 2012 William
Houston: +44 (0)20 7845 1138 John Harney: +44 (0)20 7845 1263
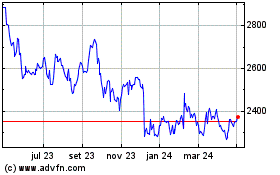
British American Tobacco (LSE:BATS)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
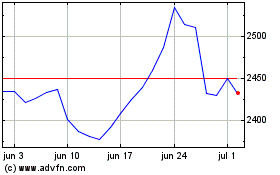
British American Tobacco (LSE:BATS)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024