Janssen Says Guselkumab Psoriatic Arthritis Studies Meet Endpoints
14 Junho 2019 - 9:36AM
Dow Jones News
By Michael Dabaie
Johnson & Johnson's (JNJ) Janssen Pharmaceutical Cos. said
the Phase 3 Discover 1 and 2 studies, which evaluated guselkumab
compared to placebo in adult patients with active moderate to
severe psoriatic arthritis, met their primary endpoints.
The studies met the goal of American College of Rheumatology 20%
improvement, and the safety profiles observed for guselkumab in the
program were consistent with previous studies of guselkumab and
current prescribing information, Janssen said.
Data from the two studies will serve as the basis of submissions
to the U.S. Food and Drug Administration and European Medicines
Agency seeking approval of guselkumab as a treatment for psoriatic
arthritis, which are anticipated for later this year.
Write to Michael Dabaie at michael.dabaie@wsj.com
(END) Dow Jones Newswires
June 14, 2019 08:21 ET (12:21 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
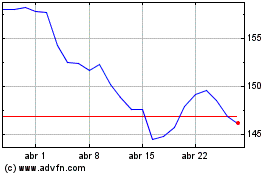
Johnson and Johnson (NYSE:JNJ)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
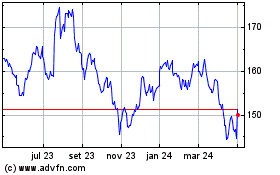
Johnson and Johnson (NYSE:JNJ)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024