Janssen Says FDA Approves Darzalex Combination Regimen in Multiple Myeloma
27 Junho 2019 - 4:18PM
Dow Jones News
By Michael Dabaie
Johnson & Johnson's (JNJ) Janssen Pharmaceutical Cos. said
Thursday that the U.S. Food and Drug Administration approved
Darzalex in combination with lenalidomide and dexamethasone for
patients with newly diagnosed multiple myeloma who are ineligible
for autologous stem cell transplant.
The approval is based on results from a Phase 3 clinical study,
which showed the combination regimen reduced the risk of disease
progression or death by 44% in newly diagnosed patients who are
transplant ineligible, Janssen said.
This is the sixth Darzalex FDA-approved use in multiple myeloma
and the second for newly diagnosed patients, the company said.
Write to Michael Dabaie at michael.dabaie@wsj.com
(END) Dow Jones Newswires
June 27, 2019 15:03 ET (19:03 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
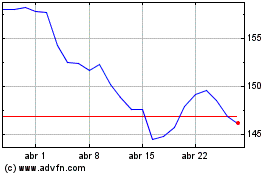
Johnson and Johnson (NYSE:JNJ)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
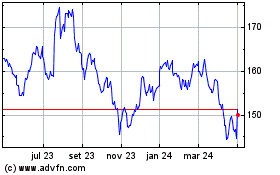
Johnson and Johnson (NYSE:JNJ)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024