OPKO Health Up 12% After Study Data
21 Outubro 2019 - 9:59AM
Dow Jones News
By Michael Dabaie
OPKO Health Inc. (OPK) shares were up 12%, to $2.34, in
premarket trading Monday.
OPKO and Pfizer Inc. (PFE) said the Phase 3 trial evaluating
somatrogon dosed once-weekly in pre-pubertal children with
growth-hormone deficiency met its primary endpoint of
non-inferiority to daily Genotropin for injection, as measured by
annual height velocity at 12 months.
The results demonstrated the potential to reduce current dosing
frequency from once-daily to a single weekly injection, the
companies said.
In 2014, Pfizer and OPKO entered into a worldwide agreement for
the development and commercialization of somatrogon for the
treatment of GHD. OPKO is responsible for conducting the clinical
program and Pfizer is responsible for registering and
commercializing the product.
Pfizer shares were up 0.6% premarket, to $36.66.
Write to Michael Dabaie at michael.dabaie@wsj.com
(END) Dow Jones Newswires
October 21, 2019 08:44 ET (12:44 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
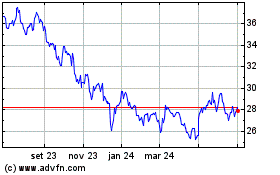
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
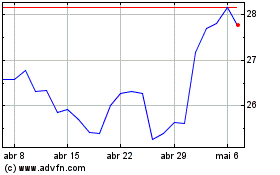
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024