Pfizer Gets Positive CHMP Opinion for Vyndaqel in ATTR-CM
13 Dezembro 2019 - 11:12AM
Dow Jones News
By Colin Kellaher
Pfizer Inc. (PFE) on Friday said the European Medicines Agency's
Committee for Medicinal Products for Human Use recommended approval
Vyndaqel for the treatment of wild-type or hereditary transthyretin
amyloidosis in adults with cardiomyopathy, or ATTR-CM.
The New York drug maker said Vyndaqel, if approved by the
European Commission, would be the first pharmacologic therapy in
the EU for ATTR-CM, a rare, life-threatening disease characterized
by the buildup of abnormal deposits of misfolded protein called
amyloid in the heart.
The European Commission, which generally follows the CHMP's
recommendations, will review the recommendation, with a final
decision expected in the coming months, Pfizer said.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
December 13, 2019 08:57 ET (13:57 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
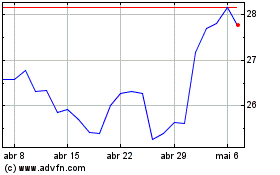
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
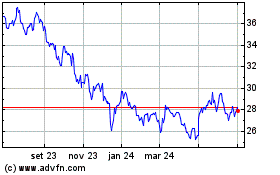
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024