Pfizer Gets Positive CHMP Opinion for Abrocitinib in Atopic Dermatitis
15 Outubro 2021 - 9:38AM
Dow Jones News
By Michael Dabaie
Pfizer Inc. said the European Medicines Agency's Committee for
Medicinal Products for Human Use recommended the 100 mg and 200 mg
doses of abrocitinib for marketing authorization to treat moderate
to severe atopic dermatitis in adults.
The CHMP also gave a positive opinion recommending marketing
authorization for Xeljanz 5 mg and 10 mg, administered twice daily,
for the treatment of adults with chronic, inflammatory disease
ankylosing spondylitis.
Based on the CHMP recommendations, a decision by the European
Commission, which authorizes marketing approval in the European
Union, is expected on the abrocitinib and Xeljanz applications
later this year, Pfizer said.
If granted by the European Commission, the centralized marketing
authorizations would be valid in all EU Member states as well as in
Iceland, Liechtenstein, and Norway.
Write to Michael Dabaie at michael.dabaie@wsj.com
(END) Dow Jones Newswires
October 15, 2021 08:23 ET (12:23 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
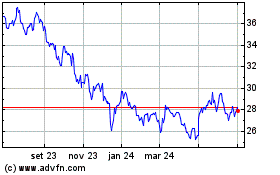
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
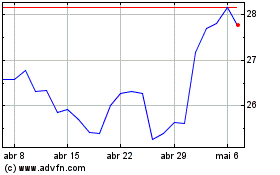
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024