Futura Medical Shares Higher After ED Treatment Update
20 Dezembro 2021 - 6:55AM
Dow Jones News
By Michael Susin
Futura Medical PLC shares rose on Monday after it said it
remains on track to submit its MED3000 erectile-dysfunction
treatment to the U.S. drug regulator by the end of the third
quarter of 2022.
Shares at 0900 GMT were up 3.30 pence, or 10%, at 34.90
pence.
The U.K. pharmaceutical developer focused on sexual health and
pain relief said it is carrying out additional studies for the U.S.
Food and Drug Administration as well as a short study about the
ability of individuals to self-diagnose their erectile dysfunction
and select the treatment correctly.
The company said it is aiming for an FDA authorization to sell
MED3000 without a doctor's prescription in the first quarter of
2023, becoming the first ED treatment available over the
counter.
"We are continuing to make good progress with the regulatory
process for MED3000 in the U.S. We are also executing upon our
strategic plans to leverage commercialization globally with a
network of licensing and distribution partners with brand-building
strength, healthcare credibility and regional infrastructure and
marketing expertise," Chief Executive James Barder said.
Write to Michael Susin at michael.susin@wsj.com
(END) Dow Jones Newswires
December 20, 2021 04:40 ET (09:40 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
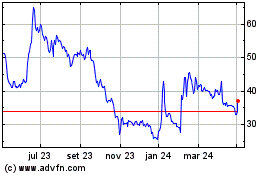
Futura Medical (LSE:FUM)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
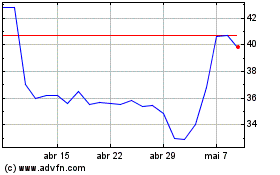
Futura Medical (LSE:FUM)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024