Canadian Regulator Approves Use of Pfizer Oral Drug for Covid-19
17 Janeiro 2022 - 1:38PM
Dow Jones News
By Adriano Marchese
Pfizer Canada said Monday that Canada's health regulator has
approved the use of its Covid-19 oral treatment for adults over the
age of 18.
The pharmaceutical and biotechnology company said that Health
Canada has authorized the use of PAXLOVID, also known as the
nirmatrelvir tablets and ritonavir tablets, for the treatment of
mild-to-moderate Covid-19.
The drug is designed to block the activity of one of the virus's
key enzymes, which it uses to replicate itself.
Health Canada approved the drug based on data from Pfizer's
phase 2/3 EPIC-HR trial for adults with Covid-19 at a higher risk
of seeing their illness progress.
The company noted that additional phase 2/3 trials are still in
process for adults at standard risk.
In December, Pfizer said it had reached an agreement with the
Canadian government to supply 1 million treatment courses of the
treatment in 2022.
"Pfizer is ready to begin delivery in Canada immediately to help
get PAXLOVID into the hands of appropriate patients as quickly as
possible," Pfizer Canada Hospital Business Unit Lead Kevin Mohamed
said.
Write to Adriano Marchese at adriano.marchese@wsj.com
(END) Dow Jones Newswires
January 17, 2022 11:23 ET (16:23 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
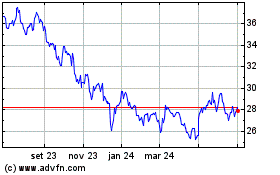
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
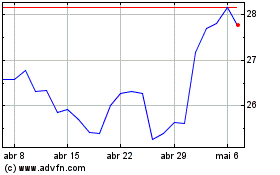
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024