Moderna Files With EU to Expand CMA for Covid-19 Vaccine
29 Abril 2022 - 1:23PM
Dow Jones News
By Stephen Nakrosis
Biotechnology company Moderna Inc. on Friday said it filed with
the European Medicines Agency to request its Spikevax Covid-19
vaccine be authorized for children aged six months to under six
years old.
Moderna said similar requests are underway with international
regulatory authorities, based on a two-dose primary series of
mRNA-1273, the research name used for Spikevax.
The company said its filing follows a Feb. 24 decision by the
EMA's Committee for Medicinal Products for Human Use "to adopt a
positive opinion recommending marketing authorization for Moderna's
Covid-19 vaccine to include children six years of age and
older."
According to Moderna, positive interim results from a Phase 2/3
study showed a "robust neutralizing antibody response in the six
months to under six years of age group after a two-dose primary
series of mRNA-1273, along with a favorable safety profile."
Write to Stephen Nakrosis at stephen.nakrosis@wsj.com
(END) Dow Jones Newswires
April 29, 2022 12:08 ET (16:08 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
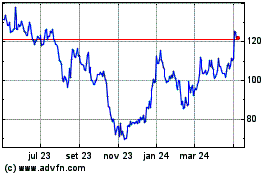
Moderna (NASDAQ:MRNA)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
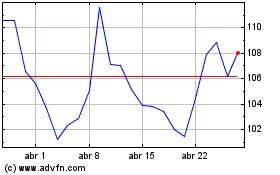
Moderna (NASDAQ:MRNA)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024