AstraZeneca's Enhertu Approved in US for Metastatic Breast Cancer Treatment
05 Maio 2022 - 4:03AM
Dow Jones News
By Michael Susin
AstraZeneca PLC said on Thursday that its metastatic breast
cancer treatment Enhertu has been approved for use in the U.S.
The Anglo-Swedish pharma giant said Enhertu will be used in
patients with breast cancer that were treated with a prior
treatment and have developed disease recurrence during or within
six months of completing therapy.
The company said clinical studies showed that the treatment has
reduced the risk of disease progression or death by 72% when
compared with other treatment.
Enhertu its a jointly treatment developed and commercialized by
AstraZenecaa and Daiichi Sankyo Co.
Astrazeneca said regulatory applications are currently under
review in Europe, Japan and several other countries.
Write to Michael Susin at michael.susin@wsj.com
(END) Dow Jones Newswires
May 05, 2022 02:48 ET (06:48 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
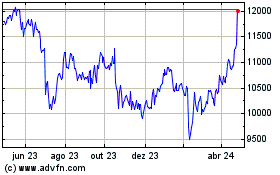
Astrazeneca (LSE:AZN)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
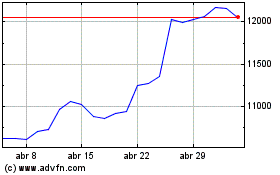
Astrazeneca (LSE:AZN)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024