Pfizer Sees Positive Results in Etrasimod Trials
24 Maio 2022 - 8:41AM
Dow Jones News
By Chris Wack
Pfizer Inc. saw positive results from two studies that make up
the Phase 3 registrational program evaluating ulcerative colitis
treatment etrasimod, the company said Tuesday.
Both Phase 3 trials achieved all primary and key secondary
endpoints, with etrasimod demonstrating a safety profile consistent
with previous studies, the company said.
In the 52-week study, clinical remission was 27% for patients
receiving etrasimod compared with 7.4% for patients receiving
placebo at week 12, and was 32.1% compared with 6.7% at week
52.
In the 12-week study, clinical remission was achieved among
24.8% of patients receiving etrasimod compared with 15.2% of
patients receiving placebo.
The 52-week trial showed statistically significant improvements
were attained in all key secondary endpoints. All key secondary
endpoints were also met at week 12 in the 12-week trial.
Treatment-emergent adverse events were similar between treatment
groups in both trials.
Etrasimod, a once-daily, oral, selective sphingosine 1-phosphate
receptor modulator, was developed by Arena Pharmaceuticals, which
was recently acquired by Pfizer.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
May 24, 2022 07:26 ET (11:26 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
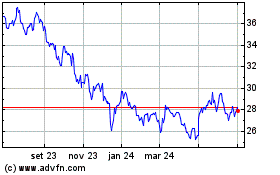
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
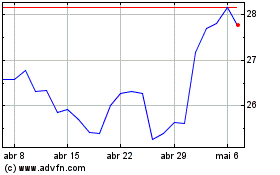
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024