Pfizer Handed $75.6 Million Fine for Overcharging UK Health Service for Epilepsy Drug
22 Julho 2022 - 5:37AM
Dow Jones News
By Joe Hoppe
Pharma giant Pfizer Inc. has been fined 63.0 million pounds
($75.6 million) by the U.K. markets regulator for overcharging the
country's National Health Service for a life-saving epilepsy
drug.
Flynn Pharma Ltd. was also fined GBP6.7 million for charging
what the Competition and Markets Authority described as unfairly
high prices for phenytoin sodium capsules for more than four years,
with the ultimate cost borne by the NHS, the regulator said in a
statement dated Thursday.
The CMA said the companies debranded the drug--formerly known as
Epanutin--so that it was no longer subject to price regulation and
allowing them to set prices at their discretion. As Pfizer and
Flynn were the dominant U.K. suppliers of the drug at the time, the
NHS had no choice but to pay the inflated final price, the
regulator said.
Over four years Pfizer charged prices between 780% and 1,600%
higher than previously, the CMA said. The company supplied the drug
to Flynn, which sold the capsules on to pharmacies and wholesalers
for 2,300%-2,600% higher than previously, the regulator said.
NHS annual costs for phenytoin capsules rose from GBP2 million
in 2012 to around GBP50 million the following year, according to
the regulator.
Neither Pfizer nor Flynn Pharma were immediately available for
comment.
Write to Joe Hoppe at joseph.hoppe@wsj.com
(END) Dow Jones Newswires
July 22, 2022 04:22 ET (08:22 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
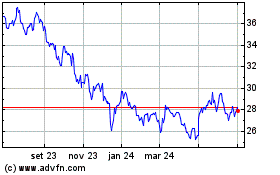
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
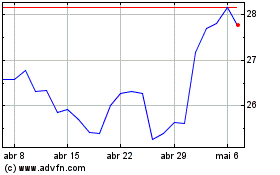
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024