AEterna Zentaris Regains Rights to Macrilen from Novo Nordisk
29 Agosto 2022 - 10:07AM
Dow Jones News
By Adriano Marchese
AEterna Zentaris Inc. said Monday that Novo Nordisk Healthcare
AG terminated its development and commercialization license
agreement for an oral test used to diagnosis adult growth hormone
deficiency known as Macrilen.
The dual-listed specialty biopharmaceutical company said it
would regain full Canadian and U.S. rights to Macrilen, also known
as macimorelin, following Novo's 270-day notice period.
In the meantime, Novo is expected to continue selling and
promoting Macrilen in the U.S. and to maintain its financial
support for current trials.
The license agreement for Macrilen was originally entered
between Novo and AEterna's wholly owned subsidiary.
AEterna said it plans to identify new strategic development and
commercialization partners.
"We are delighted to regain full control over macimorelin in the
US and Canada. We have always strongly believed in the value of
macimorelin and remain fully committed to our plans to continue
commercialization in the U.S. and Canada as well as continuing the
pediatric development in our ongoing DETECT-trial," Chief Executive
Officer Klaus Paulini said.
Write to Adriano Marchese at adriano.marchese@wsj.com
(END) Dow Jones Newswires
August 29, 2022 08:52 ET (12:52 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
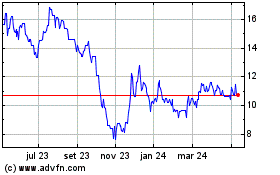
Aeterna Zentaris (TSX:AEZS)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
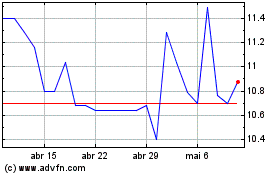
Aeterna Zentaris (TSX:AEZS)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024