Futura Medical Seeks US Approval for Erectile-Dysfunction Treatment
03 Outubro 2022 - 5:58AM
Dow Jones News
By Michael Susin
Futura Medical PLC said Monday that it will submit an
application to the U.S. Food and Drug Administration for its
topical gel treatment for erectile dysfunction.
The U.K. pharmaceutical developer focused on sexual health and
pain relief said it remains confident of receiving U.S. marketing
authorization for the treatment, or MED3000, by the end of the
first quarter of 2023.
If marketing authorization is granted, MED3000 will become the
first major erectile-dysfunction treatment available without a
doctor's prescription in the U.S., the company said.
Shares at 0812 GMT were up 0.02 pence, or 5.3%, at 0.39
pence.
Write to Michael Susin at michael.susin@wsj.com
(END) Dow Jones Newswires
October 03, 2022 04:43 ET (08:43 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
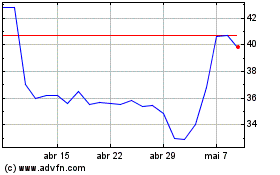
Futura Medical (LSE:FUM)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
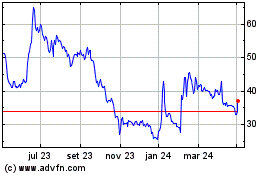
Futura Medical (LSE:FUM)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024