Pfizer's Prostate Cancer Drug Trial Meets Key Target
04 Outubro 2022 - 8:45AM
Dow Jones News
By Dean Seal
Pfizer Inc. said the Phase 3 trial studying its drug Talzenna as
a treatment for metastatic castration-resistant prostate cancer
reached its primary endpoint, with significant improvement shown in
radiographic progression-free survival.
The trial compared the use of Talzenna and Xtandi, another of
Pfizer's prostate cancer medications, with the use of a placebo and
Xtandi.
Pfizer said Tuesday that results showed a trend toward improved
overall survival, which was a key secondary endpoint, though the
company noted that the data were not yet mature at the time of
analysis. Other secondary endpoints are still being analyzed.
Detailed results will be submitted for presentation at a
near-term medical congress, Pfizer said.
Write to Dean Seal at dean.seal@wsj.com
(END) Dow Jones Newswires
October 04, 2022 07:30 ET (11:30 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
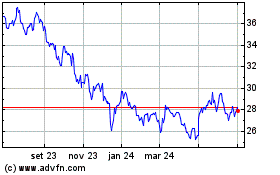
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
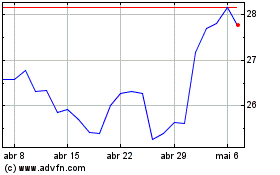
Pfizer (NYSE:PFE)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024