Abbott Molecular's Monkeypox Diagnostic Test Gets FDA Emergency Use Authorization
07 Outubro 2022 - 6:24PM
Dow Jones News
By Stephen Nakrosis
The U.S. Food and Drug Administration on Friday said it issued
an emergency use authorization to Abbott Molecular Inc. for its
monkeypox diagnostic test.
The authorization was issued for the Alinity m MPXV, a test
intended to detect monkeypox DNA using lesion swab specimens from
individuals suspected of infection, the FDA said.
The test is intended for use by qualified and trained clinical
laboratory personnel, and testing is limited to certain
laboratories, the FDA said.
According to the Centers for Disease Control, as of Sept. 30,
there have been 26,049 cases of monkeypox reported in the U.S.
Write to Stephen Nakrosis at stephen.nakrosis@wsj.com
(END) Dow Jones Newswires
October 07, 2022 17:09 ET (21:09 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
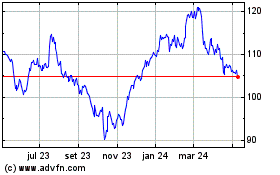
Abbott Laboratories (NYSE:ABT)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
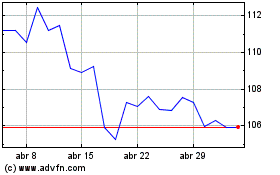
Abbott Laboratories (NYSE:ABT)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024