Abbott Gets Expanded FDA OK of Proclaim XR in Diabetic Peripheral Neuropathy
26 Janeiro 2023 - 11:48AM
Dow Jones News
By Colin Kellaher
Abbott Laboratories on Thursday said the U.S. Food and Drug
Administration approved the expanded use of its Proclaim XR
spinal-cord-stimulation system to treat painful diabetic peripheral
neuropathy.
The Abbott Park, Ill., maker of healthcare products said the
system can provide relief to patients with the debilitating
complication of diabetes who are in need of alternatives to
traditional treatment approaches, such as oral medication.
Abbott said about 34.2 million Americans, or more than 10% of
the U.S. population, have diabetes, and that roughly half of adults
with diabetes will develop peripheral neuropathy, which may include
symptoms such as pain and numbness in the legs, feet and hands. The
are currently no disease modifying treatments for diabetic
peripheral neuropathy.
The FDA in 2019 approved the Proclaim XR for the treatment of
chronic pain.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
January 26, 2023 09:33 ET (14:33 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
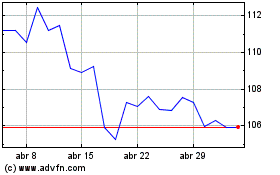
Abbott Laboratories (NYSE:ABT)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
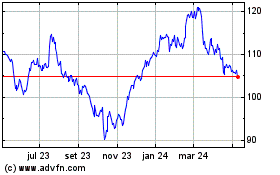
Abbott Laboratories (NYSE:ABT)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024