Futura Medical Shares Rise on Response to US Regulator for MED3000
29 Março 2023 - 6:21AM
Dow Jones News
By Joe Hoppe
Futura Medical PLC shares rose Wednesday after it said it has
submitted a full response to the U.S. Food and Drug
Administration's questions on its MED3000 gel formulation for the
treatment of erectile dysfunction.
Shares at 0847 GMT were up 4.45 pence, or 10% at 46.95
pence.
The pharmaceutical company said is has also sent the requested
confirmatory data, which together with the response, has been
resubmitted to enable the FDA to continue its review of
MED3000.
The company had said on March 14 that the FDA has asked
additional questions and requested some nonclinical confirmatory
data related to its application for marketing authorization for
MED3000.
"Based on the FDA's published target review period guidelines
which includes time to review the newly provided information, the
board continue to anticipate grant of the... request in [the second
quarter of] 2023," the company said.
Write to Joe Hoppe at joseph.hoppe@wsj.com
(END) Dow Jones Newswires
March 29, 2023 05:06 ET (09:06 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
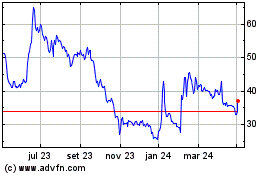
Futura Medical (LSE:FUM)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
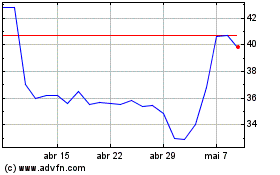
Futura Medical (LSE:FUM)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024