AstraZeneca's Ultomiris Recommended for Marketing Authorization in EU
03 Abril 2023 - 3:48AM
Dow Jones News
By Michael Susin
AstraZeneca PLC said Monday that its treatment for patients with
neuromyelitis optica spectrum disorder has been recommended for
marketing authorization in the European Union.
The pharma giant said Ultomiris has received a positive opinion
from the European Medicines Agency's Committee for Medicinal
Products for Human Use based on Phase 3 trial results.
The company said Ultomiris, which is designed for patients with
neuromyelitis optica spectrum disorder who are anti-aquaporin-4
antibody positive, has met its primary endpoint.
If authorized, the treatment would be the first and only
approved long-acting complement inhibitor for the treatment in the
European Union.
The company's regulatory submissions for Ultomiris are also
currently under review with multiple health authorities, including
in the U.S. and Japan.
Write to Michael Susin at michael.susin@wsj.com
(END) Dow Jones Newswires
April 03, 2023 02:33 ET (06:33 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
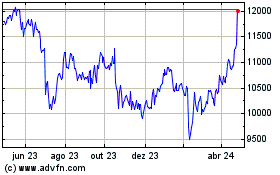
Astrazeneca (LSE:AZN)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
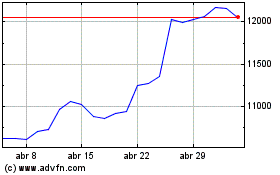
Astrazeneca (LSE:AZN)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024