AstraZeneca's Lynparza, Imfinzi Ovarian Cancer Treatment Met Primary Endpoint in Phase 3 Trial
05 Abril 2023 - 3:42AM
Dow Jones News
By Joe Hoppe
AstraZeneca PLC said Wednesday that its Lynparza and Imfinzi
combination met its endpoint in the treatment of patients with
advanced ovarian cancer.
The pharma major said the combination improved progression-free
survival in newly diagnosed patients with advanced ovarian cancer
without tumor BRCA mutations, in a DUO-O Phase 3 trial.
Positive high-level results from a planned interim analysis of
the trial showed treatment with a combination of Lynparza, Imfinzi,
chemotherapy and bevacizumab demonstrated a statistically
significant and clinically meaningful improvement in
progression-free survival when compared to just chemotherapy and
bevacizumab, it said.
In an additional arm, Imfinzi, chemotherapy and bevacizumab
without Lynparza showed a numerical improvement in progression free
survival, but didn't reach statistical significance at the interim
point.
The safety and tolerability of the combinations are broadly
consistent with observations from prior clinical trials and the
known profiles of the medicines.
The Phase 3 trial enrolled more than 1,200 patients across all
treatment arms, in a randomized, double-blind placebo-controlled
test.
Write to Joe Hoppe at joseph.hoppe@wsj.com
(END) Dow Jones Newswires
April 05, 2023 02:27 ET (06:27 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
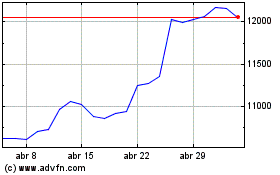
Astrazeneca (LSE:AZN)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
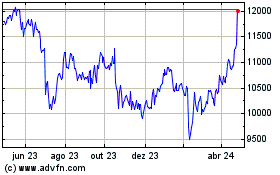
Astrazeneca (LSE:AZN)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024