Merck KGaA Shares Fall After US Puts Hold on New Patients in MS Drug Trial
12 Abril 2023 - 4:54AM
Dow Jones News
By Joshua Kirby
Shares in Germany's Merck KGaA slipped Wednesday after the
company said U.S. authorities have enforced a pause on initiating
new patients in a drug trial.
The study will continue with its current participants as
planned, the drugmaker said.
The U.S. Food & Drug Administration, or FDA, placed the
partial clinical hold on the initiation of new patients in the
Phase 3 study of evobrutinib, Merck KGaA said.
The hold also applies to patients with less than 70 days'
exposure to evobrutinib, which is being developed by Merck KGaA as
a potential treatment for relapsing multiple sclerosis.
At 0718 GMT, shares were trading 5.1% down at EUR164.10.
The pause follows two cases of apparent drug-induced liver
injury identified during the study, Merck KGaA said. Both patients
were asymptomatic and required no medical intervention or
hospitalization, the company said.
The study will continue as planned, with all participants to
remain on the treatment and results expected in the fourth quarter
this year, Merck KGaA said.
"Merck is working closely with the FDA to establish the best
path forward for the benefit of patients in current and future
trials with evobrutinib," the company said.
Write to Joshua Kirby at joshua.kirby@wsj.com;
@joshualeokirby
(END) Dow Jones Newswires
April 12, 2023 03:39 ET (07:39 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
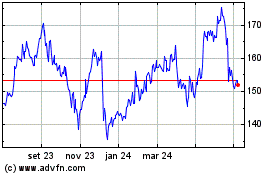
Merck KGAA (TG:MRK)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
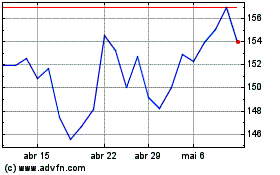
Merck KGAA (TG:MRK)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024