AstraZeneca to Stop Intracranial Hemorrhage Drug Trial After Criteria Met
05 Junho 2023 - 3:56AM
Dow Jones News
By Elena Vardon
AstraZeneca said Monday that a phase four trial for its Andexxa
drug for patients experiencing intracranial haemorrhage will be
stopped early after it achieved prespecified criteria showing an
ability to limit the expansion of potentially life-threatening
brain bleeding.
The Anglo-Swedish pharma giant said the decision was taken by
the independent data and safety monitoring board after an
assessment of efficacy with 450 patients who had been randomized
and followed for one month.
The company will now begin the closure of the trial and go ahead
with regulatory filings in the U.S. and the European Union to
convert it from conditional to full label approval.
Write to Elena Vardon at elena.vardon@wsj.com
(END) Dow Jones Newswires
June 05, 2023 02:41 ET (06:41 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
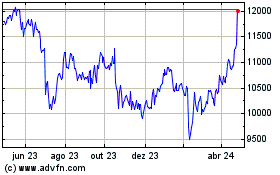
Astrazeneca (LSE:AZN)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
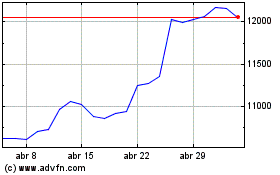
Astrazeneca (LSE:AZN)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024