AstraZeneca Cancer Drug Enhertu Shows Clinically Meaningful, Durable Response in Trial
05 Junho 2023 - 9:53AM
Dow Jones News
By Elena Vardon
AstraZeneca said Monday that the phase II trial for its cancer
drug Enhertu showed clinically meaningful and durable responses
across patients with HER2-expressing advanced solid tumours who had
been previously treated.
The Anglo-Swedish pharma giant said interim results from its
DESTINY-PanTumor02 Phase 2 trial showed an objective response rate
of 37.1% in the trial's overall population and also demonstrated
positive antitumor activity in patients previously treated for
HER2-positive metastatic colorectal cancer.
Enhertu, or trastuzumab deruxtecan, is being jointly developed
and commercialized by AstraZeneca and Daiichi Sankyo.
Write to Elena Vardon at elena.vardon@wsj.com
(END) Dow Jones Newswires
June 05, 2023 08:38 ET (12:38 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
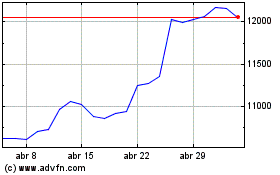
Astrazeneca (LSE:AZN)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
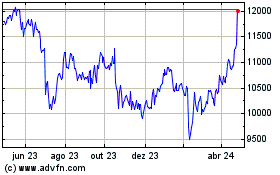
Astrazeneca (LSE:AZN)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024