Futura Medical PLC MED3000, Eroxon(R) UK Launch (4464W)
18 Abril 2023 - 3:00AM
UK Regulatory
TIDMFUM
RNS Number : 4464W
Futura Medical PLC
18 April 2023
18 April 2023
Futura Medical plc
("Futura" or the "Company")
MED3000, Eroxon (R) UK Launch
Futura Medical plc (AIM: FUM) ("Futura" or the "Company"), a
pharmaceutical company developing a portfolio of innovative
products based on its proprietary, transdermal DermaSys(R) drug
delivery technology currently focused on sexual health, today
announces that its breakthrough, topical gel formulation for the
treatment of erectile dysfunction (ED), MED3000 , under the brand
name Eroxon (R), is now available for purchase in the UK.
Futura's EU and UK distribution partner, Cooper Consumer Health,
a leading International independent self-care organisation, has
today announced that Eroxon(R) is now available from boots.com and
is being rolled out in store from 18(th) April 2023.
The prevalence of ED disrupts the lives of at least 1 in 5 men
globally with around 22 million men suffering ED in the US and 20
million men in the UK, France, Italy, Spain and Germany. ED is a
growing problem, with around half of men over the age of 40
experiencing ED and a quarter of men diagnosed with ED now being
under the age of 40. This is being driven by an ageing population,
increased obesity and prevalence of health conditions such as
diabetes which contribute to erectile problems, as well as societal
pressures and "performance anxiety" issues in the case of younger
men. There has been little innovation in ED treatments in over a
decade and many patients continue to suffer dissatisfaction with
existing treatments.
Eroxon(R), which is fast-acting with minimal side-effects, has
the opportunity to provide an alternative option and significant
benefits compared to existing ED treatments, especially for those
patients seeking a spontaneous intercourse experience as Eroxon(R)
helps users get an erection within 10 minutes.
MED3000 is the first pan-European topical treatment for ED
available without the need of a doctor's prescription and available
over the counter.
James Barder, Chief Executive Officer of Futura Medical said:
"We are pleased to confirm that Eroxon is available in the UK from
today. This is a significant milestone for the Company as our
distribution partners continue to increase the availability of
Eroxon(R) across different markets. ED is a growing problem and we
believe that today's announcement will be welcomed by many men
suffering from ED for whom current front-line treatments for ED are
unsuitable."
ENDS
For further information please contact:
Futura Medical plc
James Barder, Chief Executive Officer
Angela Hildreth, Finance Director and COO
Email: investor.relations@futuramedical.com
Tel: +44 (0) 1483 685 670
www.futuramedical.com
Nominated Adviser and Sole Broker:
Liberum
Phil Walker/ Richard Lindley/ Ben Cryer
Tel: +44 (0) 20 3100 2000
For media enquiries please contact:
Optimum Strategic Communications
Mary Clark/ Hollie Vile/ Jonathan Edwards/ Zoe Bolt
Email: futuramedical@optimumcomms.com
Tel: +44 (0) 203 882 9621
About Futura Medical plc
Futura Medical plc (AIM: FUM), is a pharmaceutical company
developing a portfolio of innovative products based on its
proprietary, transdermal DermaSys(R) technology. Each DermaSys(R)
formulation is separately patented and specifically tailored for
the selected indication and application, as well as being optimised
for clinical efficacy, safety, administration and patient
convenience. The products are developed for the prescription and
consumer healthcare markets as appropriate.. Development and
commercialisation strategies are designed to maximise product
differentiation and value creation whilst minimising risk.
MED3000 is Futura's topical gel formulation that is a novel
treatment for erectile dysfunction ("ED") through a unique
evaporative mode of action. Futura has previously conducted an
initial Phase 3 study using MED3000 in ED, referred to as "FM57"
which enabled Futura to be granted a CE Mark in 2021. A second
confirmatory Phase 3 clinical study, "FM71" was also conducted to
support Futura's regulatory submission to the FDA with 96 ED
patients and endpoints at 24 weeks, demonstrating that MED3000
presents an effective clinically proven treatment for ED with a
rapid speed of onset and a favourable benefit versus risk profile
ideally suited for an 'Over the Counter' classification.
Eroxon(R) is CE marked in Europe and UKCA marked in the UK as a
clinically proven topical treatment for adult men with erectile
dysfunction under the brand Eroxon(R) with a key claim of "Helps
you get an erection within 10 minutes". Eroxon(R) is the agreed
brand name in certain regions such as the EU whereas MED3000
continues to be the internal code name used by the Company and also
in reference to countries where regulatory approval or commercial
distribution agreements have not yet been achieved.
www.eroxon.com
Futura is based in Guildford, Surrey, and its shares trade on
the AIM market of the London Stock Exchange.
www.futuramedical.com
This information is provided by RNS, the news service of the
London Stock Exchange. RNS is approved by the Financial Conduct
Authority to act as a Primary Information Provider in the United
Kingdom. Terms and conditions relating to the use and distribution
of this information may apply. For further information, please
contact rns@lseg.com or visit www.rns.com.
RNS may use your IP address to confirm compliance with the terms
and conditions, to analyse how you engage with the information
contained in this communication, and to share such analysis on an
anonymised basis with others as part of our commercial services.
For further information about how RNS and the London Stock Exchange
use the personal data you provide us, please see our Privacy
Policy.
END
MSCFLFSISLIDLIV
(END) Dow Jones Newswires
April 18, 2023 02:00 ET (06:00 GMT)
Futura Medical (LSE:FUM)
Gráfico Histórico do Ativo
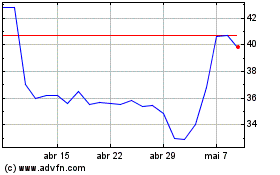
De Mar 2024 até Abr 2024
Futura Medical (LSE:FUM)
Gráfico Histórico do Ativo
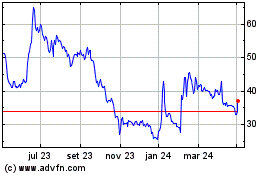
De Abr 2023 até Abr 2024