Novartis Unit Sandoz Says Biosimilar Aflibercept Mylight Phase III Study Met Endpoint
15 Agosto 2023 - 3:11AM
Dow Jones News
By Pierre Bertrand
Novartis unit Sandoz said on Tuesday that its Mylight Phase III
study assessing the efficacy of its biosimilar aflibercept drug met
its primary endpoint.
The Mylight study showed the drug had no clinically meaningful
differences to reference aflibercept.
The biosimilar drug could be a treatment option for patients
with neovascular age-related macular degeneration, a leading cause
of visual impairment and progressive vision loss for older adult,
Sandoz said.
The company added that it will file biosimilar aflibercept for
regulatory approval in the coming months in both the U.S. and the
European Union.
Write to Pierre Bertrand at pierre.bertrand@wsj.com
(END) Dow Jones Newswires
August 15, 2023 01:56 ET (05:56 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
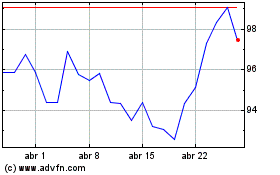
Novartis (NYSE:NVS)
Gráfico Histórico do Ativo
De Abr 2024 até Mai 2024
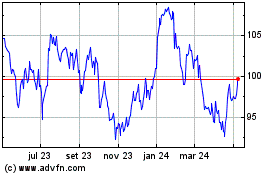
Novartis (NYSE:NVS)
Gráfico Histórico do Ativo
De Mai 2023 até Mai 2024