Vertex Pharmaceuticals Gets Regulatory OK to Extend Use to More Patients in Europe
24 Novembro 2023 - 12:06PM
Dow Jones News
By Adriano Marchese
Vertex Pharmaceuticals said on Friday that a European regulator
validated a new variation application to the marketing
authorization for its cystic fibrosis treatment.
The pharmaceutical company said the European Medicines Agency
has validated a Type II variation application for Kaftrio, also
known as ivacaftor, tezacaftor and elexacaftor, in combination with
ivacaftor.
On Thursday, Vertex said the European Commission granted
approval for the label expansion of the treatment for children
between the ages of two and five with cystic fibrosis.
The application, which aims to expand the treatment's use to
include people with cystic fibrosis and responsive rare mutations,
is now set to be reviewed by the Committee for Medicinal Products
for Human Use. That committee will issue its opinion to the
European Commission regarding the potential approval of this
license expansion.
Backing the treatment is data from a Phase 3 clinical study,
which met its endpoint and showed that the treatment resulted in
statistically significant improvements over the placebo.
Vertex said that it also includes real-world evidence data from
a patient registry of people with cystic fibrosis in the U.S.
Write to Adriano Marchese at adriano.marchese@wsj.com
(END) Dow Jones Newswires
November 24, 2023 09:51 ET (14:51 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
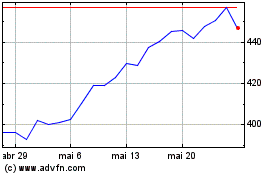
Vertex Pharmaceuticals (NASDAQ:VRTX)
Gráfico Histórico do Ativo
De Mai 2024 até Jun 2024
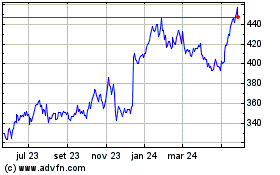
Vertex Pharmaceuticals (NASDAQ:VRTX)
Gráfico Histórico do Ativo
De Jun 2023 até Jun 2024