CEL-SCI Announces Pricing of $10.8 Million Offering
26 Julho 2024 - 12:52PM
Business Wire
CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American:
CVM), a cancer immunotherapy company, today announced the pricing
of a best-efforts offering of 10,845,000 shares of its common stock
(or pre-funded warrants (“Pre-Funded Warrants”) in lieu thereof).
Each share of common stock (or Pre-Funded Warrant) is being sold at
an offering price of $1.00 per share (inclusive of the Pre-Funded
Warrant exercise price). All of the shares and Pre-Funded Warrants
in the offering are being offered by the Company. Total gross
proceeds from the offering, before deducting the placement agent’s
fees and other offering expenses, are expected to be $10,845,000.
The offering is expected to close on July 29, 2024, subject to
satisfaction of customary closing conditions.
The Company intends to use the net proceeds from the offering to
fund the continued development of Multikine, general corporate
purposes, and working capital.
ThinkEquity is acting as sole placement agent for the
offering.
The securities will be offered and sold pursuant to a shelf
registration statement on Form S-3 (File No. 333-265995), including
a base prospectus, filed with the U.S. Securities and Exchange
Commission (the “SEC”) on July 1, 2022, and declared effective on
July 15, 2022. The offering will be made only by means of a written
prospectus. A prospectus supplement and accompanying prospectus
describing the terms of the offering will be filed with the SEC on
its website at www.sec.gov. Copies of the prospectus supplement and
the accompanying prospectus relating to the offering may also be
obtained, when available, from the offices of ThinkEquity, 17 State
Street, 41st Floor, New York, New York 10004.
This press release shall not constitute an offer to sell or a
solicitation of an offer to buy, nor shall there be any sale of
these securities in any state or jurisdiction in which such an
offer, solicitation or sale would be unlawful prior to registration
or qualification under the securities laws of any such state or
jurisdiction.
About CEL-SCI
Corporation
CEL-SCI is a clinical-stage biotechnology company focused on
finding the best way to activate the immune system to fight cancer
and infectious diseases. The Company’s lead investigational therapy
Multikine completed a pivotal Phase 3 clinical trial involving head
and neck cancer, for which the Company has received Orphan Drug
Status from the FDA. The Company will be commencing a confirmatory
trial for Multikine, with enrollment expected to begin in Q4 2024.
The Company has operations in Vienna, Virginia, and near Baltimore,
Maryland. https://cel-sci.com/
Forward-Looking
Statements
This press release contains forward-looking statements within
the meaning of Section 27A of the Securities Act of 1933, as
amended, and Section 21E of the Securities Exchange Act of 1934, as
amended. When used in this press release, the words "intends,"
"believes," "anticipated," "plans" and "expects," and similar
expressions, are intended to identify forward-looking statements.
Such statements are subject to risks and uncertainties that could
cause actual results to differ materially from those projected.
Such statements include, but are not limited to, statements about
the terms, expected proceeds, use of proceeds and closing of the
offering. Factors that could cause or contribute to such
differences include an inability to duplicate the clinical results
demonstrated in clinical studies, timely development of any
potential products that can be shown to be safe and effective,
receiving necessary regulatory approvals, difficulties in
manufacturing any of the Company's potential products, inability to
raise the necessary capital and the risk factors set forth from
time to time in CEL-SCI's filings with the Securities and Exchange
Commission, including but not limited to its report on Form 10-K
for the year ended September 30, 2023. The Company undertakes no
obligation to publicly release the result of any revision to these
forward-looking statements which may be made to reflect the events
or circumstances after the date hereof or to reflect the occurrence
of unanticipated events.
* Multikine (Leukocyte Interleukin, Injection) is the trademark
that CEL-SCI has registered for this investigational therapy. This
proprietary name is subject to FDA review in connection with the
Company's future anticipated regulatory submission for approval.
Multikine has not been licensed or approved for sale, barter or
exchange by the FDA or any other regulatory agency. Similarly, its
safety or efficacy has not been established for any use.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240726229829/en/
For Investor Relations Inquiries: Gavin de Windt CEL-SCI
Corporation (703) 506-9460
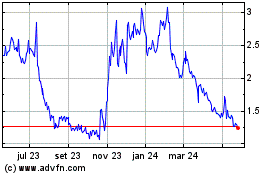
Cel Sci (AMEX:CVM)
Gráfico Histórico do Ativo
De Jan 2025 até Fev 2025
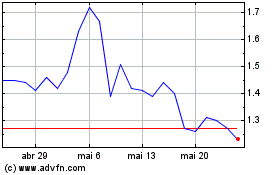
Cel Sci (AMEX:CVM)
Gráfico Histórico do Ativo
De Fev 2024 até Fev 2025