Vivoryon Therapeutics N.V. Reports Positive Outcome from Independent Data Safety Monitoring Board with Unanimous Decision for VIVA-MIND U.S. Study of Varoglutamstat in Alzheimer’s Disease to Proceed at Highest Investigated Dose
23 Outubro 2023 - 2:00AM

Vivoryon Therapeutics N.V. Reports Positive Outcome from
Independent Data Safety Monitoring Board with Unanimous Decision
for VIVA-MIND U.S. Study of Varoglutamstat in Alzheimer’s Disease
to Proceed at Highest Investigated Dose
Vivoryon Therapeutics N.V. Reports
Positive Outcome from Independent Data Safety Monitoring Board with
Unanimous Decision for VIVA-MIND U.S. Study of Varoglutamstat in
Alzheimer’s Disease to Proceed at Highest Investigated
Dose
- Interim analysis
completed, with 600mg twice daily dosing unanimously selected by
independent DSMB as final dose for remainder of Phases 2a and 2b of
VIVA-MIND study in U.S.
- Decision follows
2022 DSMB determination for VIVIAD Phase 2 EU study to proceed at
600mg twice daily based on safety and tolerability of 90 patients
followed for at least 24 weeks post randomization
Halle (Saale) / Munich, Germany, October
23, 2023 – Vivoryon Therapeutics N.V. (Euronext Amsterdam:
VVY; NL00150002Q7) (Vivoryon), a clinical stage
company focused on the discovery and development of small molecule
medicines to modulate the activity and stability of pathologically
altered proteins, today announced that the study’s independent data
safety monitoring board (DSMB) unanimously recommend that the
VIVA-MIND study of varoglutamstat should proceed with a dose of
600mg twice daily (BID) through the remainder of Phases 2a and
2b.
VIVA-MIND (NCT03919162) is an ongoing Phase 2
study for varoglutamstat conducted in the U.S., complementary to
Vivoryon’s VIVIAD Phase 2b study being conducted in Europe.
VIVA-MIND seeks to enroll 180 patients with early Alzheimer’s
disease (AD) into the Phase 2a adaptive dose finding portion and
enroll a further 234 patients in the Phase 2b portion of the study.
In July 2023, the Company announced that the first cohort of the
study was fully randomized as planned and is now recruiting
participants into the second cohort with 21 sites open across the
U.S. The primary endpoint of the study is evaluating Clinical
Dementia Rating scale Sum of Boxes (CDR-SB) over a 72-week
treatment period.
“We are thrilled to report this new important
and validating element of our rigorously and meticulously designed
program for varoglutamstat. While all prior DSMB decisions have
been favorable, we believe that this marks a critical next step on
our path towards consciously determining the optimal dosing regimen
for varoglutamstat. From the initial stages of determining the
maximum tolerated dose in our completed SAPHIR Phase 2a study,
through the extremely encouraging interim analyses of tolerability
in our European VIVIAD Phase 2b study, we keep making key steps
towards optimizing safety. At the same time, we are striving to
ensure we can maintain high levels of target occupancy to maximize
our probability of success in improving not only memory, but many
other important elements of cognition in those suffering from mild
cognitive impairment and early AD,” said Frank Weber, M.D., CEO of
Vivoryon. “We have designed two complimentary studies intended to
support the efficient advancement of varoglutamstat towards
regulatory approval. At our recent R&D Event, multiple
perspectives from renowned Key Opinion Leaders further elucidated
the solid base of understanding for the potential of varoglutamstat
to treat early AD patients, in particular the endpoints in VIVIAD
and VIVA-MIND, which we are confident will provide a deep
understanding of varoglutamstat’s effect on relevant cognitive
outcome parameters and patient function in separate sensitive and
validated scales. Taken together, we believe that our unique
approach has the potential to dramatically improve the lives of
millions of people whose daily lives are impacted by AD.“
“I am very pleased to have received the
unanimous recommendation of the independent DSMB of the VIVA-MIND
trial to continue the highest investigated dose arm of 600mg BID
for the remainder of the study. Importantly, the trial has now
passed the milestone of an interim evaluation of a predetermined
safety boundary of specified adverse events,” commented Dr. Howard
Feldman, Professor of Neurosciences, Co-Director of The Alzheimer's
Disease Cooperative Study (ADCS) at the University of California
San Diego School of Medicine, and the VIVA-MIND Principal
Investigator. “As varoglutamstat progresses further through its
Phase 2a/b design, we will continue to gather further safety and
tolerability data, of this oral treatment and its profile which may
differentiate from anti-amyloid-antibodies which are delivered by
infusion.“
The DSMB’s dose decision recommending that the
highest dose of varoglutamstat of 600mg BID be selected for the
remainder of the trial follows its September 18, 2023 quarterly
safety review of adverse events, and labs, and its October 19, 2023
analysis of treatment-emergent adverse events of special interest
(AESI) pertaining to skin and subcutaneous tissue disorders and
hepatobiliary disorders, as well as target occupancy and plasma
pharmacokinetic (PK) data.
In July 2023, Vivoryon announced that it
commenced preparations for an open-label extension (OLE) study to
provide a long-term treatment option to patients after completion
of treatment under the VIVIAD or VIVA-MIND protocol. The launch of
the OLE study is contingent on the outcome of VIVIAD. Vivoryon
remains on track to report the final data readout from the VIVIAD
study in the first quarter of 2024.
###
About VIVA-MINDVIVA-MIND is a
complementary Phase 2 study being conducted in the U.S.,
coordinated by the Alzheimer's Disease Cooperative Study (ADCS) at
the University of California San Diego (UCSD) School of Medicine
and supported by the National Institute on Aging (NIA), part of the
National Institutes of Health (NIH) with a $15 million grant (NIA
award number R01AG061146). The study seeks to enroll 180 patients
into the Phase 2a adaptive dose-finding portion with the Phase 2b
portion, enrolling an additional 234 patients treated at the
selected dose for at least 72 weeks, with a total of 414 patients
being treated on stable doses of varoglutamstat for 18 months. The
VIVA-MIND design was adapted in 2022 to enable all 180 patients in
the Phase 2a portion to be treated for at least 72 weeks, allowing
for the opportunity to progress seamlessly to a potential Phase 3
study. The flexible study design is aimed at increasing the
probability of success by broadening option space for adjustments
in clinical development based on learnings from VIVIAD and other
developments in the field. The primary endpoint for this study is
clinical dementia rating scale - sum of boxes (CDR-SB), an
established approvable endpoint measuring a combination of
cognitive abilities and activities of daily living. Secondary
efficacy endpoints include quantitative EEG theta power, ADAS-Cog
13 and others. Exploratory endpoints include mini-mental state
examination (MMSE), Montreal cognitive assessment (MoCA),
quantitative EEG alpha power, relative QPCT activity in CSF and
others.
About Vivoryon Therapeutics
N.V.Vivoryon is a clinical stage biotechnology company
focused on developing innovative small molecule-based medicines.
Driven by our passion for ground-breaking science and innovation,
we strive to change the lives of patients in need suffering from
severe diseases. We leverage our in-depth expertise in
understanding post-translational modifications to develop medicines
that modulate the activity and stability of proteins which are
altered in disease settings. Beyond our lead program,
varoglutamstat, which is in Phase 2 clinical development to treat
Alzheimer’s disease, we have established a solid pipeline of orally
available small molecule inhibitors for various indications
including cancer, inflammatory diseases and fibrosis.
www.vivoryon.com
Vivoryon Forward Looking
StatementsThis press release includes forward-looking
statements, including, without limitation, those regarding the
business strategy, management plans and objectives for future
operations of the Vivoryon Therapeutics N.V. (the “Company”),
estimates and projections with respect to the market for the
Company’s products and forecasts and statements as to when the
Company’s products may be available. Words such as “anticipate,”
“believe,” “estimate,” “expect,” “forecast,” “intend,” “may,”
“plan,” “project,” “predict,” “should” and “will” and similar
expressions as they relate to the Company are intended to identify
such forward-looking statements. These forward-looking statements
are not guarantees of future performance; rather they are based on
the Management’s current expectations and assumptions about future
events and trends, the economy and other future conditions. The
forward-looking statements involve a number of known and unknown
risks and uncertainties. These risks and uncertainties and other
factors could materially adversely affect the outcome and financial
effects of the plans and events described herein. Actual results,
performance or events may differ materially from those expressed or
implied in such forward-looking statements and from expectations.
As a result, no undue reliance should be placed on such
forward-looking statements. This press release does not contain
risk factors. Certain risk factors that may affect the Company’s
future financial results are discussed in the published annual
financial statements of the Company. This press release, including
any forward-looking statements, speaks only as of the date of this
press release. The Company does not assume any obligation to update
any information or forward-looking statements contained herein,
save for any information required to be disclosed by law.
For more information, please contact:
Investor ContactStern IRJulie SeidelTel: +1
212-698-8684Email: julie.seidel@sternir.com
Media ContactTrophic CommunicationsValeria
FisherTel: +49 175 8041816Email: vivoryon@trophic.eu
- 231023_VVY_Positive Outcome from Independent DSMB
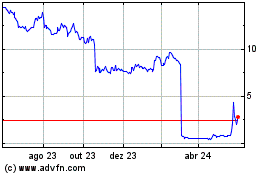
Vivoryon Therapeut (EU:VVY)
Gráfico Histórico do Ativo
De Nov 2024 até Dez 2024
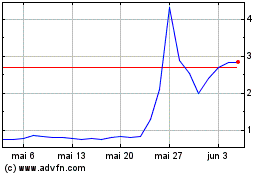
Vivoryon Therapeut (EU:VVY)
Gráfico Histórico do Ativo
De Dez 2023 até Dez 2024