AC Immune, Lilly Begin Dosing Subject with Tau Morphomer Inhibitor
17 Julho 2019 - 8:50AM
Dow Jones News
By Chris Wack
AC Immune SA (ACIU) said Wednesday it has begun dosing the first
subject in a Phase 1 study of its ACI-3024, an investigational oral
small molecule Tau Morphomer inhibitor that will be studied in
neurodegenerative diseases that are characterized by the presence
of pathological Tau aggregates.
The clinical-stage biopharmaceutical company said in a release
that this is the first significant advancement in its collaboration
with Eli Lilly & Co. (LLY).
AC Immune said ACI-3024 is the primary focus of a license and
collaboration agreement between it and Lilly to research and
develop small molecule Tau aggregation inhibitors for the treatment
of Alzheimer's disease and other neurodegenerative diseases. The
collaboration combines AC Immune's proprietary Morphomer discovery
platform and early development experience with Lilly's clinical
development expertise and commercial capabilities in central
nervous system disorders.
AC Immune said it will conduct the initial Phase 1 development
of the Morphomer Tau aggregation inhibitors while Lilly will fund
and conduct further clinical development.
AC Immune said the Phase 1 trial is a randomized dose study with
open label food effect and pharmacodynamics assessment arms to
assess the safety, tolerability, pharmacokinetics, and
pharmacodynamics of ACI-3024 in healthy volunteers.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
July 17, 2019 07:35 ET (11:35 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
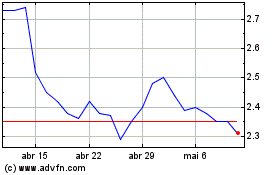
AC Immune (NASDAQ:ACIU)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
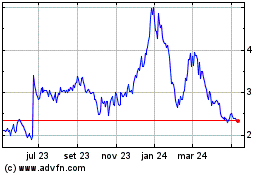
AC Immune (NASDAQ:ACIU)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024