Novartis Gets FDA Fast-Track Designation for LNA043 in Knee Osteoarthritis
02 Setembro 2021 - 7:57AM
Dow Jones News
By Colin Kellaher
Novartis AG on Thursday said the U.S. Food and Drug
Administration granted fast-track designation to LNA043 for the
treatment for osteoarthritis of the knee.
The Swiss drug maker said it is developing LNA043 as a potential
first-in-class disease-modifying treatment for osteoarthritis.
The FDA's fast-track program is designed to facilitate the
development and expedite the review of treatments for serious or
potentially life-threatening illnesses with high unmet medical
needs.
Novartis said it currently is conducting a Phase IIb study in
patients with the chronic degenerative disease that is
characterized by a progressive loss of cartilage, leading to pain,
loss of joint function and disability.
Novartis said treatment with intra-articular injections of
LNA043 in a proof-of-concept study resulted in regeneration of
damaged cartilage in patients with femoral articular cartilage
lesions.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
September 02, 2021 06:42 ET (10:42 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
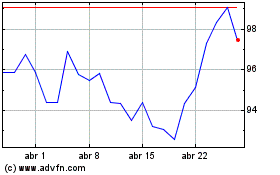
Novartis (NYSE:NVS)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
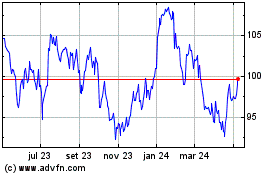
Novartis (NYSE:NVS)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024