Correction to FDA OKs Bristol Myers' Orencia Story
20 Dezembro 2021 - 11:28AM
Dow Jones News
Bristol Myers Squibb won Food and Drug Administration approval
for expanded use of its arthritis drug Orencia as the first U.S.
drug for the prevention of acute graft-versus-host disease. The
story "FDA OKs Bristol Myers' Orencia in Acute Graft-Vs-Host
Disease", published on Dec. 15, inaccurately described the final
indication.
(END) Dow Jones Newswires
December 20, 2021 09:13 ET (14:13 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
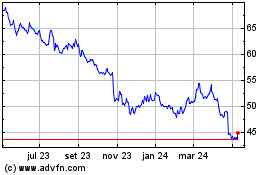
Bristol Myers Squibb (NYSE:BMY)
Gráfico Histórico do Ativo
De Abr 2024 até Mai 2024
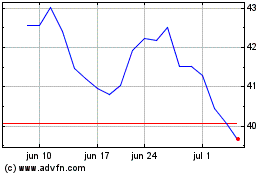
Bristol Myers Squibb (NYSE:BMY)
Gráfico Histórico do Ativo
De Mai 2023 até Mai 2024