Novartis's Vijoice Gets U.S. Nod
06 Abril 2022 - 2:32AM
Dow Jones News
By Joshua Kirby
Novartis AG said Wednesday that its drug Vijoice (alpelisib) has
been granted accelerated approval in the U.S. for the treatment of
adults and children with severe manifestations of PIK3CA-Related
Overgrowth Spectrum (PROS) who require systemic therapy.
The U.S. Food & Drug Administration gave the approval to
Vijoice for adults and children above two years of age suffering
from the PROS, a spectrum of rare conditions characterized by
atypical overgrowths and anomalies in blood vessels, the lymphatic
system and other tissues, the Swiss drugmaker said.
The accelerated approval program means continued approval may be
contingent upon proof of clinical benefit from confirmatory
evidence, Novartis said.
Write to Joshua Kirby at joshua.kirby@wsj.com;
@joshualeokirby
(END) Dow Jones Newswires
April 06, 2022 01:17 ET (05:17 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
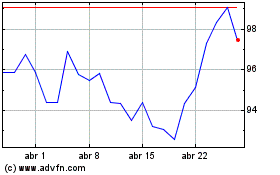
Novartis (NYSE:NVS)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
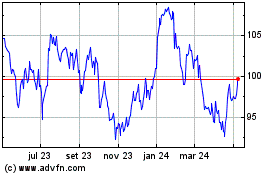
Novartis (NYSE:NVS)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024