Eagle Pharmaceuticals Gets Fast-Track Designation for Pneumonia Treatment
14 Junho 2023 - 8:39AM
Dow Jones News
By Dean Seal
Eagle Pharmaceuticals said regulators have granted fast-track
designation for CAL02, its non-biological bacterial virulence
neutralizer and anti-infective agent that's being developed to
treat severe community-acquired bacterial pneumonia as an add-on
therapy.
Chief Executive Scott Tarriff said Wednesday that the
designation allows it to work more closely with the U.S. Food and
Drug Administration to advance the new treatment option, as it
would be eligible to request a priority review of its new drug
application.
The FDA has also granted a Qualified Infectious Disease Product
designation for CAL02, the company said.
With that designation, Eagle expects to have eight to 10 years
of regulatory exclusivity once its new drug application gets
approved.
Write to Dean Seal at dean.seal@wsj.com
(END) Dow Jones Newswires
June 14, 2023 07:24 ET (11:24 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
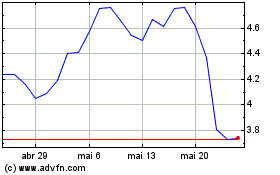
Eagle Pharmaceuticals (NASDAQ:EGRX)
Gráfico Histórico do Ativo
De Abr 2024 até Mai 2024
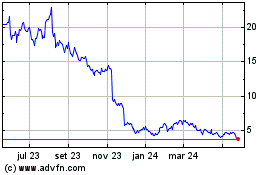
Eagle Pharmaceuticals (NASDAQ:EGRX)
Gráfico Histórico do Ativo
De Mai 2023 até Mai 2024