0001513525
false
0001513525
2023-09-26
2023-09-26
0001513525
us-gaap:CommonStockMember
2023-09-26
2023-09-26
0001513525
ADIL:WarrantsMember
2023-09-26
2023-09-26
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities
Exchange Act of 1934
Date of Report (date of earliest event reported):
September 26, 2023
Adial Pharmaceuticals, Inc.
(Exact name of registrant as specified in charter)
| Delaware |
|
001-38323 |
|
82-3074668 |
(State or other jurisdiction
of incorporation) |
|
(Commission File
Number) |
|
(IRS Employer
Identification No.) |
1180 Seminole Trail, Ste 495
Charlottesville, VA 22901
(Address of principal executive offices and zip
code)
(434) 422-9800
(Registrant’s telephone number including
area code)
N/A
(Former name or former address, if changed
since last report)
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of registrant under any of the following provisions (see General
Instruction A.2. below):
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12(b) under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading Symbols |
|
Name of each exchange on which registered |
| Common Stock |
|
ADIL |
|
The Nasdaq Stock Market LLC
(Nasdaq Capital Market) |
| |
|
|
|
|
| Warrants |
|
ADILW |
|
The Nasdaq Stock Market LLC
(Nasdaq Capital Market) |
Indicate by check mark whether the registrant
is an emerging growth company as defined in in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of
the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by checkmark
if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards
provided pursuant to Section 13(a) of the Exchange Act.
Item 8.01. Other Events.
Adial Pharmaceuticals, Inc. (the “Company”) is filing this
Current Report on Form 8-K, including Exhibit 99.1, solely to recast certain financial information and related disclosures included in
the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022 originally filed with the U.S. Securities and Exchange
Commission (the “SEC”) on March 30, 2023 (the “2022 Form 10-K”).
On June 30, 2023, the Company completed the sale
of the assets and business of Purnovate, Inc. (“Purnovate”) to Adovate, LLC (formerly known as Adenomed, LLC) under that Option
Agreement for the Acquisition of Purnovate, Inc. by Adenomed, LLC, dated as of January 27, 2023, and related Option Exercise Agreement,
dated May 8, 2023. For all periods presented in this Form 8-K, the historical results of operations of the former Purnovate and its assets
and liabilities have been reflected as discontinued operations in our recast consolidated financial statements for all periods presented
in Exhibit 99.1, including, as of December 31, 2022 and 2021, the assets and liabilities associated with Purnovate being classified as
assets and liabilities of discontinued operations in our consolidated balance sheets.
This Form 8-K is being filed solely to recast
financial information and related disclosures contained in the 2022 Form 10-K to reflect the sale of the recast business of Purnovate.
The following items of the 2022 Form 10-K are being recast (collectively, the “Revised Sections”) as reflected in Exhibit 99.1
to this Current Report on Form 8-K.
| ● | Part II, Item 7. Management’s Discussion and Analysis of
Financial Condition and Results of Operations; |
| ● | Part II, Item 8. Financial Statements and Supplementary Data. |
The disclosures in the Revised Sections were updated
to reflect the Company’s results as if the discontinued operations criteria for Purnovate had been met during the periods being
presented. Other than Note 13 of Part II, Item 8 “Financial Statements and Supplementary Data,” the Revised Sections, including
Management’s Discussion and Analysis of Financial Condition and Results of Operations and the Company’s Business Outlook contained
therein, do not reflect events occurring after the filing of the 2022 Form 10-K and do not modify or update the disclosures therein in
any way for any information, uncertainties, transactions, risks, events or trends occurring, or known to management, other than as specifically
required to reflect the discontinued operations presentation. Other than the Revised Sections filed with this Current Report on Form 8-K,
all other information in the 2022 Form 10-K remains unchanged and has not been otherwise updated for events occurring after the filing
of the 2022 Form 10-K, and continues to speak only as of the original filing date.
More recent information, including risk factors,
is contained in the Company’s quarterly reports on Form 10-Q for the quarterly periods ended March 31, 2023 (the “Q1 Form 10-Q”)
and June 30, 2023 (the “Q2 Form 10-Q”) and other filings made by the Company with the SEC. Such filings contain important information
regarding events, developments, and updates affecting the Company and its expectations, including those that have occurred since the filing
of the 2022 Form 10-K. Accordingly, this Current Report on Form 8-K should be read in conjunction with the Q1 Form 10-Q, Q2 Form 10-Q
and other filings made by the Company with the SEC subsequent to the filing of the 2022 Form 10-K.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
The following exhibits are furnished with this
Current Report on Form 8-K:
* * *
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Dated: September 26, 2023 |
ADIAL PHARMACEUTICALS, INC. |
| |
|
| |
By: |
/s/ Cary J. Claiborne |
| |
Name: |
Cary J. Claiborne |
| |
Title: |
President and Chief Executive Officer |
2
Exhibit 23.1
Independent
Registered Public Accounting Firm’s Consent
We consent to the incorporation by reference in
the Registration Statements of Adial Pharmaceuticals, Inc. on Form S-8 (File No. 333-226884, 333-233760, 333-248759, 333-260304 and 333-267972),
Form S-3 (File No. 333-237793, 333-255352, 333-256621, 333-258048, 333-261509 and 333-263037), and Form S-1 (File No. 333-251122, 333-239678,
and 333-272846) of our report dated September 26, 2023, which includes an explanatory paragraph as to the Company’s ability to continue
as a going concern, with respect to our audits of the consolidated financial statements as of and for the years ended December 31, 2022
and 2021, which report is included in this Current Report on Form 8-K of Adial Pharmaceuticals, Inc.
/s/ Marcum llp
Marcum llp
Marlton, New Jersey
September 26, 2023
Exhibit 99.1
Item 7. Management’s Discussion and Analysis of Financial
Condition and Results of Operations.
The following discussion and analysis is intended
as a review of significant factors affecting our financial condition and results of operations for the periods indicated. The discussion
should be read in conjunction with our consolidated financial statements and the notes presented herein. In addition to historical information,
the following Management’s Discussion and Analysis of Financial Condition and Results of Operations contains forward-looking statements
that involve risks and uncertainties. See “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements”
included elsewhere in the 2022 Annual report on Form 10-K. Our actual results could differ significantly from those expressed, implied
or anticipated in these forward-looking statements as a result of certain factors discussed herein and any other periodic reports filed
and to be filed by us with the Securities and Exchange Commission.
On August 4, 2023, we effected a reverse stock
split of our outstanding shares of common stock, trading on Nasdaq under the symbol ADIL, at a ratio of 1-for-25. As a result of the reverse
split, we had 1,197,630 shares of common stock outstanding immediately after effecting the reverse split. The shares authorized for issue
under our charter remained 50,000,000 common stock. We have retrospectively adjusted all references to common stock, stock warrants to
purchase common stock, stock options to purchase common stock, share data, per share data and related information contained in the following
discussion to reflect the effect of the reverse stock split.
Effective June 30, 2023, we sold the business
of our wholly owned subsidiary, Purnovate, Inc., to a third party. As a result, the assets, liabilities, and results of Purnovate were
classified as discontinued operations. We have retrospectively reclassified all assets, liabilities, and results of Purnovate as discontinued
operations in the following discussion and have adjusted all references to Purnovate assets, liabilities, and results accordingly.
Overview
We are a clinical-stage
biopharmaceutical company focused on the development of therapeutics for the treatment or prevention of addiction and related disorders.
Our lead investigational new drug product, AD04, is a genetically targeted therapeutic agent being developed for the treatment of alcohol
use disorder (“AUD”). AD04 was recently investigated in a Phase 3 clinical trial, designated the ONWARD trial, for the potential
treatment of AUD in subjects with certain target genotypes, which were identified using our companion diagnostic genetic test. Based on
our analysis of the subgroup data from the ONWARD trial, we are now focused on commercializing AD04 in the U.S. and Europe.
We continue to explore
opportunities to expand our portfolio in the field of addiction and related disorders, both through internal development and through acquisitions.
Our vision is to create the world’s leading addiction focused pharmaceutical company.
In January 2021, we expanded our portfolio in
the field of addiction with the acquisition of Purnovate, LLC via a merger into our wholly owned subsidiary, Purnovate, Inc., (“Purnovate”)
and in January 2023, we entered into an option agreement with Adovate, LLC (formerly Adenomed, LLC)(“Adovate”), pursuant to
which we granted to Adovate an exclusive option for a period of one hundred twenty (120) days from the effective date of the Option Agreement
for Adovate or its designated affiliate to acquire the business and assets of Purnovate. Effective May 16, 2023, Adovate exercised its
option and paid the upfront and exercise fees. Effective June 30, 2023, Adovate issued to the Company the equity stake in Adovate due
on exercise of the option agreement, acquired Purnovate’s business and assets, and the transaction was closed.
We have devoted the vast majority of our resources
to development efforts relating to AD04, including preparation for conducting clinical trials, providing general and administrative support
for these operations and protecting our intellectual property.
We currently do not have any products approved
for sale and we have not generated any significant revenue since our inception. From our inception through the date of our 2022 Annual
Report on Form 10-K, we have funded our operations primarily through the private and public placements of debt, equity securities, and
an equity line.
Our current cash and cash equivalents are not
expected to be sufficient to fund operations for the twelve months from the date of filing our 2022 Annual report on Form 10-K, based
our current projections.
We have incurred net losses in each year since
our inception, including net losses of approximately $12.7 million and $19.4 million for the years ended December 31, 2022 and 2021. We
had accumulated deficits of approximately $63.7 and $50.9 million as of December 31, 2022 and 2021, respectively. Most, about 85%, of
our operating losses resulted from costs incurred in continuing operations, including costs in connection with our continuing research
and development programs, from general and administrative costs associated with our operations, and from financing costs.
We will not generate revenue from product sales
unless and until we successfully complete development and obtain marketing approval for AD04, which we expect will take a number of years
and is subject to significant uncertainty. We do not believe our current cash and equivalents will be sufficient to fund our operations
for the next twelve months from the filing of these financial statements.
Until such time, if ever, as we can generate substantial
revenue from product sales, we expect to finance our operating activities through a combination of equity offerings, debt financings,
government or other third-party funding, commercialization, marketing and distribution arrangements and other collaborations, strategic
alliances and licensing arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed
on favorable terms or at all. Our failure to raise capital or enter into such other arrangements as and when needed would have a negative
impact on our financial condition and our ability to develop AD04.
Clinical Trials — Research and
Development Schedule
We currently anticipate that we, working in collaboration
with our vendors, upon execution of collaborative research and development agreements with them, will be able to execute the following
timeline:
AD04 — Two-Stage Clinical Development Strategy — Conduct
the Phase 3 clinical trials sequentially
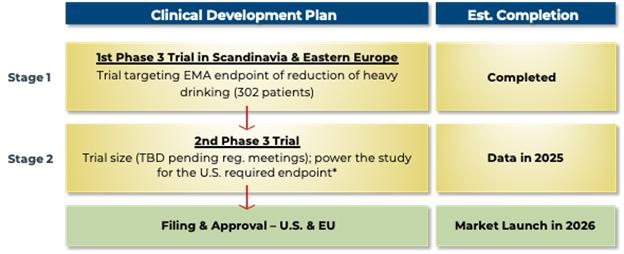
| * |
Even if the 1st Phase 3 trial is not accepted by the FDA due to the study not being well-powered for the FDA’s currently stated end point, we still expect that the EMA will require only one additional trial. In this case, however, a 3rd trial might be required by the FDA (i.e., three Phase 3 trials in total). If two additional trials are required for FDA approval after an initial Phase 3 trial conducted in the EMA, we would expect to run the 2nd and 3rd trials in parallel (i.e., at the same time) so as not to increase the expected time to approval. The 2nd Phase 3 trial is expected to require $8-12 million in direct expenses, and up to $5 million in additional other development expenses is expected to be required. A possible 3rd Phase 3 trial would be expected to require an additional $8-12 million in clinical trial related expenditures. |
We have completed initial Phase 3 trial, the ONWARD™
pivotal Phase 3 clinical trial using AD04 for the potential treatment of AUD in subjects with certain target genotypes.
We do not anticipate significant additional direct
expenses resulting from the ONWARD trial as of the date of the filing, though continued expenditures relating to data analysis, strategic
planning, and regulatory follow-up resulting from the trial continue.
Additional funds are expected to be raised through
grants, partnerships with other pharmaceutical companies or through additional debt or equity financings, including pursuant to the terms
of our equity line. We expect the second Phase 3 Trial, if required, to cost approximately $8-12 million, such estimate subject to the
factors stated above.
2022 and 2023 Financing Developments
On February 23, 2023, we entered into a securities
purchase agreement (the “2023 Purchase Agreement”) with an accredited institutional investor providing for the issuance of
73,170 shares (the “Shares”) of the Company’s common stock, par value $0.001 (the “Common Stock”). Pursuant
to the 2023 Purchase Agreement, the Investor purchased the Shares for an aggregate purchase price of $750,000 and expected net proceeds
of approximately $550,000. Pursuant to the 2023 Purchase Agreement, we issued an aggregate of 73,170 shares of common stock to the Investor.
On February
10, 2022, we entered into a securities purchase agreement (the “2022 Purchase Agreement”) with an accredited institutional
investor providing for the issuance of (i) 92,890 shares of Common Stock, (ii) pre-funded warrants (the “Pre-Funded Warrants”)
to purchase up to 74,600 shares of Common Stock (the “Pre-Funded Warrant Shares”) with an exercise price of $0.025 per share,
which Pre-Funded Warrants are to be issued in lieu of shares of Common Stock to ensure that the investor does not exceed certain beneficial
ownership limitations, and (iii) warrants (the “2022 Warrants”), with a term of five years and six months from the date
of issuance, to purchase an aggregate of up to 159,115 shares of Common Stock (the “2022 Warrant Shares”) at an exercise price
of $63.00 per share, subject to customary adjustments thereunder. The total net proceeds, after expenses, to us were approximately $9.1
million. All 74,600 Pre-Funded Warrants were exercised on June 8, 2022, resulting in the issue of 74,600 shares of common stock for proceeds
of $1,865.
Clinical and Research Developments
In March 2023, we announced
an update to our regulatory strategy for AD04. Key highlights included:
| |
● |
ONWARD Phase 3 clinical trial data showed that AD04 achieved a statistically significant mean reduction in heavy drinking days among the pre-specified group of “heavy drinkers” (defined as those drinking less than 10 drinks per drinking day) |
| |
● |
Additional analysis of ONWARD™ data allowed refinement of genetic panel to target specific modulators of the serotonin 3 receptor A & B subunit genotypes that outperformed others |
| |
● |
Type C meeting with the U.S. Food and Drug Administration confirmed for Q2 2023 to discuss clinical program in U.S. |
| |
● |
Meetings scheduled with two European country-level regulatory authorities and requested with three European country-level regulatory authorities |
| |
● |
Advancing discussions with potential U.S. and European partners |
| |
● |
Market research subsequent to completion of the ONWARD trial suggests unit pricing for AD04 could be significantly higher than previous assumptions |
On July 20, 2022, we announced the following results
from the Company’s ONWARD™ trial. We also announced our intent to share the results of the ONWARD trial with the relevant
health authorities to discuss the appropriate next steps towards the expeditious development of AD04 and to seek product approval.
| |
● |
AD04 patients, compared with placebo patients, achieved a statistically significant reduction from baseline at month six in heavy drinking days for the pre-specified patient group of heavy drinkers (avg. <10 drinks per drinking day at baseline; p=0.03), which accounted for approximately two-thirds of the trial population. A similar trend was seen in the combined month five and six analysis in the reduction from baseline (p =0.07). |
| |
● |
AD04 patients, compared with placebo patients, showed a trend in the reduction from baseline at month six in heavy drinking days for the combined trial population of heavy and very heavy drinkers (p=NS), which was influenced by the high placebo response among very heavy drinkers (avg. ≥10 drinks per drinking day at baseline), due to both the AD04 and placebo groups reducing mean heavy drinking days by more than 50%. A similar, non-statistically significant trend was seen in the combined months five and six analysis in the reduction from baseline, which was the pre-specified primary efficacy analysis. |
| |
● |
Compared with placebo patients, AD04 patients in the heavy drinking group had an overall significant difference in the severity of their AUD diagnosis (p=0.04) under the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). For the group of those who no longer meet AUD criteria (<2 symptoms), the comparisons were 27.4% vs. 14.9% (i.e., an 84% decrease), of AD04 and placebo patients, respectively. These data underscore the clinical relevance of the findings that heavy drinking AUD patients that receive AD04 appear more likely to recover from the disease by the end of the treatment regimen. |
| |
● |
Based on the levels of alcohol consumption reported in a meta-analysis of 83 prospective studies in primary care screening for those with AUD (Wood, et. al., Lancet 2018), the Company estimates that a majority of potential patients for AD04 would fall under the pre-specified group of heavy drinkers. This finding underscores the potential broad applicability of the results to general practice and that they could be the basis for potential regulatory approvals. |
Additionally, and consistent with results from
our Phase 2b study, AD04 had a safety and tolerability profile that was similar to placebo:
| |
● |
Serious Adverse Events (SAEs) |
| |
* |
No SAEs were determined to be related to AD04 treatment. |
| |
* |
More SAEs were reported in the placebo group compared with the AD04 group (7 on placebo vs. 3 on AD04). |
| |
* |
There were two cardiac events in placebo group and none in the AD04 group. |
| |
● |
Side effects/Adverse Events (AEs) |
| |
* |
The AE profiles between AD04 and placebo were similar. |
| |
* |
AEs reported with a frequency of 5% or more of patients in either group were: headache (11% on placebo, 12% on AD04), insomnia (3% on placebo, 7% on AD04), blood magnesium decreased (5% on placebo, 6% on AD04), and fatigue (3% on placebo, 6% on AD04). All of the above AE’s were reported as mild to moderate. |
| |
* |
Importantly, in the overall category of cardiac disorders, patients on placebo showed a greater number of adverse events relative to AD04 (7% on placebo, 4% on AD04), in addition to greater number of cardiac SAEs in the placebo group as reported above. |
Results of operations for the years ended December
31, 2022 and 2021 (rounded to nearest thousand)
The following table sets forth the components
of our statements of operations in dollars for the periods presented:
| | |
For the Year Ended
December 31, | | |
Change | |
| | |
2022 | | |
2021 | | |
(Decrease) | |
| Research and development expenses | |
$ | 1,950,000 | | |
| 7,832,000 | | |
| (5,882,000 | ) |
| General and administrative expenses | |
| 8,909,000 | | |
| 9,177,000 | | |
| (268,000 | ) |
| Total Operating Expenses | |
| 10,859,000 | | |
| 17,009,000 | | |
| (6,150,000 | ) |
| | |
| | | |
| | | |
| | |
| Loss From Operations | |
| (10,859,000 | ) | |
| (17,009,000 | ) | |
| 6,150,000 | |
| | |
| | | |
| | | |
| | |
| Interest income | |
| 63,000 | | |
| 7,000 | | |
| 56,000 | |
| Other Income | |
| – | | |
| 17,000 | | |
| 17,000 | |
| Total other expenses | |
| 63,000 | | |
| 24,000 | | |
| 39,000 | |
| Net Loss before provision for income taxes | |
| (10,796,000 | ) | |
| (16,985,000 | ) | |
| 6,189,000 | |
| Provision for income tax | |
| – | | |
| – | | |
| – | |
| Loss from continuing operations | |
| (10,796,000 | ) | |
| (16,985,000 | ) | |
| 6,189,000 | |
| Loss from discontinued operations, net of tax | |
| (1,936,000 | ) | |
| (2,439,000 | ) | |
| 503,000 | |
| Net loss | |
| (12,732,000 | ) | |
| (19,424,000 | ) | |
| 6,692,000 | |
Research and development (“R&D”) expenses
Research and development expenses decreased by
$5,882,000 (75%) during year ended December 31, 2022 compared to the year ended December 31, 2021. This decrease was due to the sharp
decrease in direct trial expenses of $5,585,000, trials insurance of $85,000, and in ONWARD-supporting manufacturing expenses of $396,000
with the completion of the ONWARD trial clinical activities during the year ended December 31, 2022, compared to the year ended December
31, 2021 when the ONWARD trial was actively recruiting patients. These decreases were partially offset by increases in post-trial regulatory
and statistical consulting of $224,000.
General and administrative expenses
The year ended December 31, 2022 saw a modest
decrease of $268,000 in G&A expenses compared to the year ended December 31, 2021, driven primarily by a large decrease in G&A
equity compensation expense of $752,000 due to decreased use of stock grants and the completed vesting of a number of options grants in
the period, substantial decreases in business development, PR, IR consultants of $305,000 as a result of management efforts to rationalize
this expense category, and modest decrease in corporate legal expenses of $55,000. These decreases were partially offset by increases
in G&A-directed salaries of $633,000 associated with increased headcounts and increased use of strategic consultants to assist management
in formulating a strategy in response to the ONWARD trial data, increasing this expense category by $247,000.
Total other income
Other income increased by approximately $39,000
in the year ended December 31, 2022 compared to the year ended December 31, 2021. This increase was due to substantially increased prevailing
interest rates in the year ended December 31, 2022 over the previous year, which allowed the company to realize approximately $56,000
in additional returns on the working capital funds it held in money market accounts. This increase was partially offset by the decrease
an approximately $17,000 in revenue realized from sales of third party produced Covid testing supplies, those sales activities being temporary
activities wound up by the end of the second quarter of 2021.
Loss from discontinued operations, net
of taxes
The loss from discontinued operations, net of
taxes, decreased by approximately $503,000 (21%) in the year ended December 31, 2022, when compared to the year ended December 31, 2021.
Expenses associated with Purnovate operations, the operations classified as discontinued due to their sale, were substantially higher
in several categories in the year ended December 31, 2022 compared to the year ended December 31, 2021, with increases of approximately
$1,641,000 in direct R&D expenses such a preclinical testing and manufacturing, approximately $80,000 in higher Purnovate personnel
expenses with increases in headcounts, approximately $61,000 in additional Purnovate supporting professional fees, and $7,000 in rent
and facilities expenses, all due to the ramping up of Purnovate’s development programs. Purnovate also did not see the recurrence
of the one-time income of approximately $29,000 as a result of the forgiveness of its PPP loan in early 2021. These increases in expenses,
however, were more than compensated for by the decrease in earn out expense of approximately $804,000 due to the change in value of the
contingent payments due the former Purnovate shareholders, and the one time charge of approximately $1,548,000 due to the impairment of
acquired Purnovate supplies that took place in the year ended December 31, 2021.
Liquidity and Capital Resources
Overview
Our principal liquidity needs have historically
been working capital, R&D, patent costs and personnel costs. We expect these needs to continue to increase in the near term as we
develop and eventually commercialize our compound, if approved. Over the next several years, we expect to increase our R&D expenses
as we undergo clinical trials to demonstrate the safety and efficacy of our lead product candidate. To date, we have funded our operations
primarily with the proceeds from our initial and secondary public offerings, private placements and our equity line, as well as other
equity financings and the issuance of debt securities prior to that. On July 31, 2018, we closed our initial public offering.
During the year ended December 31, 2022, our primary
sources of funding were sales of common stock, pre-funded warrants, and warrants, and option exercises.
On February 10, 2022, we entered into a securities
purchase agreement with an accredited institutional investor providing for the issuance of (i) 92,890 shares of our common stock, par
value $0.001, (ii) pre-funded warrants to purchase up to 74,600 shares of Common Stock with an exercise price of $0.025 per share, which
Pre-Funded Warrants were to be issued in lieu of shares of Common Stock to ensure that the Investor does not exceed certain beneficial
ownership limitations, and (iii) warrants, with a term of five years and six months from the date of issuance, to purchase an aggregate
of up to 159,115 shares of Common Stock at an exercise price of $63.00 per share. We realized net proceeds from the offering of approximately
$9.1 million after deducting fees due to the placement agent and our transaction expenses.
On February 23, 2023, we entered into an equity
purchase agreement with an accredited investor for the purchase of 73,170 shares of commons stock at at-the-market price of $10.25 per
share in a registered direct offering. We realized expected net proceeds from the offering of approximately $550,000 after deducting fees
due to the placement agent and our transaction expenses. We also issued the placement agent warrant to purchase 7,317 shares of common
stock at an exercise price of $10.25 per share.
Our current cash and cash equivalents are not
expected to be sufficient to fund operations for the twelve months from the date of filing this current report on Form 8-K, based our
current projections.
We expect to have used approximately $6.3 million
in cash during the twelve months ended December 31, 2023 for both AD04 development costs, other R&D project costs (including for operations
now classified as discontinued prior to sale), and general corporate expenses. There is no assurance that funds could be raised in that
period on acceptable terms.
We will also require additional financing as we
continue to execute our overall business strategy, including an estimated $8-12 million for a second phase three trial of AD04. Our liquidity
may be negatively impacted as a result of research and development cost increases in addition to general economic and industry factors.
Our continued operations will depend on our ability to raise additional capital through various potential sources, such as equity and/or
debt financings, grant funding, strategic relationships, or out-licensing in order to complete its subsequent clinical trial requirements
for AD04. Management is actively pursuing financing and other strategic plans but can provide no assurances that such financing or other
strategic plans will be available on acceptable terms, or at all. Without additional funding, the Company would be required to delay,
scale back or eliminate some or all of its research and development programs, which would likely have a material adverse effect on us
and our financial statements.
If we raise additional funds by issuing equity
securities or convertible debt, our shareholders will experience dilution. Debt financing, if available, would result in increased fixed
payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions, such
as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration and
licensing arrangements with third parties, it may be necessary to relinquish valuable rights to our products, future revenue streams or
product candidates or to grant licenses on terms that may not be favorable to us. We cannot be certain that additional funding will be
available on acceptable terms, or at all. Any failure to raise capital in the future could have a negative impact on our financial condition
and our ability to pursue our business strategies.
Cash flows
| | |
For the Year Ended
December 31, | |
| (rounded to nearest thousand) | |
2022 | | |
2021 | |
| Provided by (used in) | |
| | |
| |
| Operating activities – continuing operations | |
$ | (8,594,000 | ) | |
$ | (11,266,000 | ) |
| Discontinued operations | |
| (2,592,000 | ) | |
| (683,000 | ) |
| Investing activities | |
| – | | |
| (34,000 | ) |
| Financing activities | |
| 9,126,000 | | |
| 13,644,000 | |
| Net increase (decrease) in cash and cash equivalents | |
$ | (2,060,000 | ) | |
$ | 1,661,000 | |
Net cash used in operating activities – continuing operations
Net cash used in operating activities associated
with continuing operations decreased by $2,672,000 in the year ended December 31, 2022 compared to the year ended December 31, 2021. This
reduction in cash used was substantially less than the $6,150,000 decrease in operating expenses when comparing the same two periods.
This difference is due to much of the reduction in expense being non-cash based, such as $866,000 in expense decrease being reduction
in equity compensation expense. We also used $285,000 more cash to pre-pay expenses and $2,934,000 more cash to pay previously accrued
expenses in the year ended December 31, 2022 than we did in the year ended December 31, 2021.
Net cash used in discontinued operations
Net cash used in discontinued operations increased
by $1,909,000 in the year ended December 31, 2022 compared to the year ended December 31, 2021. This increase in cash used contrasts with
the $504,000 decrease to the loss from discontinued operations when comparing the same periods. This difference is primarily due to two
decreases in non-cash expenses when comparing the two periods: $1,548,000 reduced non-cash impairment charges and $804,000 being the difference
in the non-cash gain of $522,000 in 2022 and the non-cash loss in 2021 on the change in value of the contingent liability.
Net cash provided by investing activities
The Company purchased Purnovate and capital equipment
in the year ended December 31, 2021, resulting in net negative cash flow due to investing in discontinued operations of $34,000. No such
investing activity, either in continuing or discontinued operations, took place in the year ended December 31, 2022.
Net cash provided by financing activities
Net cash provided by financing activities decreased
by $4,518,000 during the year ended December 31, 2022 compared to the year ended December 31, 2021. This decrease was due to the combination
of management’s assessment that less financing would be needed to take the Company to its next critical milestone of releasing the
ONWARD trial data with generally tighter capital markets. During the year ended December 31, 2022, substantially all of our cash flows
from financing activities was from the proceeds that we received from the sale of $9,124,000 of common stock and warrants in February
2022. During the year ended December 31, 2021, our cash flows from financing activities was primarily from the sale of $11,750,000 from
shares of common stock and warrants and to a lesser extent proceeds of $1,425,000 from exercise of existing warrants.
Off-balance sheet arrangements
We do not have any off-balance sheet arrangements.
Recent Accounting Pronouncements
See Note 3 to the financial statements for a discussion
of recent accounting pronouncements.
Critical Accounting Policies and Estimates
The preparation of the financial statements requires
us to make assumptions, estimates and judgments that affect the reported amounts of assets and liabilities, our expected liquidity needs
and expected future cash positions, and the reported amounts of sales and expenses during the reporting periods. Certain of our more critical
accounting policies require the application of significant judgment by management in selecting the appropriate assumptions for calculating
financial estimates. By their nature, these judgments are subject to an inherent degree of uncertainty. On an ongoing basis, we evaluate
our judgments, including those related to prepaid research and development, accruals associated with third party providers supporting
clinical trials, realization of income tax assets, as well as the, fair value of stock based compensation to employees and service providers.
We use historical experience and other assumptions as the basis for our judgments and making these estimates. Because future events and
their effects cannot be determined with precision, actual results could differ significantly from these estimates. Any changes in those
estimates will be reflected in our financial statements as they occur.
While our significant accounting policies are
more fully described in Note 3 to our financial statements included elsewhere in our 2022 Annual Report on Form 10-K, we believe that
the following accounting policies and estimates are most critical to a full understanding and evaluation of our reported financial results.
R&D Expenses
Recognition and accrual of expenses associated
with our clinical trial are dependent on the judgment of our contractors and subcontractors in their reporting and communication of information
to us. Occurrence of certain fees to our CRO, clinical trial sites, and subcontractors are tied to events, for which the determination
of likelihood requires judgment both on our part and on the part of our contractors.
Stock Based Compensation
We estimate the fair value of options and stock
warrants granted using the Black Scholes Merton model. We estimate when and if performance-based awards will be earned. If an award is
not considered probable of being earned, no amount of equity-based compensation expense is recognized. If the award is deemed probable
of being earned, related equity-based compensation expense is recorded. The fair value of an award ultimately expected to vest is recognized
as an expense, net of forfeitures, over the requisite service periods in our statements of operations, which is generally the vesting
period of the award.
The Black Scholes Merton model requires the input
of certain subjective assumptions and the application of judgment in determining the fair value of the awards. The most significant assumptions
and judgments include the expected volatility, risk-free interest rate, the expected dividend yield, and the expected term of the awards.
In addition, the recognition of equity-based compensation expense is impacted by our forfeitures, which are accounted for as they occur.
The assumptions used in our option pricing model
represent management’s best estimates. If factors change and different assumptions are used, our equity-based compensation expense
could be materially different in the future. The key assumptions included in the model are as follows:
| |
● |
Expected volatility — We determine the expected price volatility based on the historical volatilities of a peer group as we do not have a sufficient trading history for our units. Industry peers consist of several public companies in the bio-tech industry similar to us in size, stage of life cycle and financial leverage. We intend to continue to consistently apply this process using the same or similar public companies until a sufficient amount of historical information regarding the volatility of our own stock price becomes available, or unless circumstances change such that the identified companies are no longer similar to us, in which case, more suitable companies whose share prices are publicly available would be utilized in the calculation. Starting in 2020, we have begun blending data on our historical volatility together with this peer group of companies, the proportion of our volatility used growing as the period of our historical volatility becomes longer. |
| |
● |
Risk-free interest rate — The risk free rate was determined based on yields of U.S. Treasury Bonds of comparable terms. |
| |
● |
Expected dividend yield — We have not previously issued dividends and do not anticipate paying dividends in the foreseeable future. Therefore, we used a dividend rate of zero based on our expectation of additional dividends. |
| |
● |
Expected term —The expected term of the options was estimated using the simplified method. |
Commitments and Contingencies
We follow subtopic 450-20 of the FASB Accounting
Standards Codification to report accounting for contingencies. Certain conditions may exist as of the date the financial statements are
issued, which may result in a loss to us but which will only be resolved when one or more future events occur or fail to occur. We assess
such contingent liabilities, and such assessment inherently involves an exercise of judgment.
If the assessment of a contingency indicates that
it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would
be accrued in our financial statements. If the assessment indicates that a potentially material loss contingency is not probable but is
reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, and an estimate of the range
of possible losses, if determinable and material, would be disclosed.
Loss contingencies considered remote are generally
not disclosed unless they involve guarantees, in which case the guarantees would be disclosed. Our legal costs associated with contingent
liabilities are recorded to expense as incurred.
Item 8. Financial Statements and Supplementary
Data
ADIAL PHARMACEUTICALS, INC.
FINANCIAL STATEMENTS
Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
To the Shareholders and Board of Directors of
Adial Pharmaceuticals, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated
balance sheets of Adial Pharmaceuticals, Inc. (the “Company”) as of December 31, 2022 and 2021, the related consolidated statements
of operations, stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2022, and the related
notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in
all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its
cash flows for each of the two years in the period ended December 31, 2022, in conformity with accounting principles generally accepted
in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying financial statements have been
prepared assuming that the Company will continue as a going concern. As more fully described in Note 2, the Company has a significant
working capital deficiency, has incurred significant losses and needs to raise additional funds to meet its obligations and sustain its
operations. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in
regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the
outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We
are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are
required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and
regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the
standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial
statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged
to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding
of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal
control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess
the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond
to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating
the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Emphasis of Matter
As discussed in Note 3 and Note 4, the accompanying
consolidated financial statements reflect retrospective application of the reverse stock split and of the discontinued operations.
/s/ Marcum llp
We have served as the Company’s auditor since 2017 (such date
takes into account the acquisition of certain assets of Friedman LLP by Marcum LLP effective September 1, 2022)
Marlton, New Jersey
September 26, 2023
ADIAL PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
| | |
December 31,
2022 | | |
December 31,
2021 | |
| ASSETS | |
| | |
| |
| Current Assets: | |
| | |
| |
| Cash and cash equivalents | |
$ | 4,001,794 | | |
$ | 6,062,173 | |
| Prepaid research and development | |
| — | | |
| 9,931 | |
| Prepaid expenses and other current assets | |
| 349,441 | | |
| 389,501 | |
| Current assets of discontinued operations | |
| 428,700 | | |
| — | |
| Total Current Assets | |
| 4,779,935 | | |
| 6,461,605 | |
| | |
| | | |
| | |
| Intangible assets, net | |
| 4,477 | | |
| 5,041 | |
| Non-Current Assets of discontinued operations | |
| 948,392 | | |
| 1,008,329 | |
| Total Assets | |
$ | 5,732,804 | | |
$ | 7,474,975 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |
| | | |
| | |
| Current Liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 276,410 | | |
$ | 261,288 | |
| Accrued expenses | |
| 963,327 | | |
| 2,376,930 | |
| Accrued expenses, related party | |
| 175,000 | | |
| — | |
| Other current liabilities | |
| 10,387 | | |
| 9,683 | |
| Current liabilities of discontinued operations | |
| 365,742 | | |
| 74,489 | |
| Total Current Liabilities | |
| 1,790,866 | | |
| 2,722,390 | |
| | |
| | | |
| | |
| Long-term Liabilities: | |
| | | |
| | |
| Long-term liabilities of discontinued operations | |
| 665,444 | | |
| 1,244,774 | |
| Total Liabilities | |
$ | 2,456,310 | | |
$ | 3,967,164 | |
| | |
| | | |
| | |
| Commitments and contingencies – see Note 9 | |
| | | |
| | |
| | |
| | | |
| | |
| Stockholders’ Equity | |
| | | |
| | |
| Preferred Stock, 5,000,000 shares authorized with a par value of $0.001 per share, 0 shares outstanding at December 31, 2022 and 2021 | |
| — | | |
| — | |
| Common Stock, 50,000,000 shares authorized with a par value of $0.001 per share, 1,067,491 and 837,868 shares issued and outstanding at December 31, 2022 and 2021, respectively | |
| 1,067 | | |
| 838 | |
| Additional paid in capital | |
| 66,949,958 | | |
| 54,450,088 | |
| Accumulated deficit | |
| (63,674,531 | ) | |
| (50,943,115 | ) |
| Total Stockholders’ Equity | |
| 3,276,494 | | |
| 3,507,811 | |
| Total Liabilities and Stockholders’ Equity | |
$ | 5,732,804 | | |
$ | 7,474,975 | |
The accompanying notes are an integral part of
these consolidated financial statements.
ADIAL PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| | |
For the Years Ended
December 31, | |
| | |
2022 | | |
2021 | |
| Operating Expenses: | |
| | |
| |
| Research and development | |
$ | 1,950,308 | | |
$ | 7,832,000 | |
| General and administrative | |
| 8,909,167 | | |
| 9,176,641 | |
| Total Operating Expenses | |
| 10,859,475 | | |
| 17,008,641 | |
| Loss From Operations | |
| (10,859,475 | ) | |
| (17,008,641 | ) |
| Other Income | |
| | | |
| | |
| Interest income | |
| 63,338 | | |
| 6,539 | |
| Other income | |
| — | | |
| 17,406 | |
| Total other income | |
| 63,338 | | |
| 23,945 | |
| Loss Before Provision For Income Taxes | |
| (10,796,137 | ) | |
| (16,984,696 | ) |
| Income tax benefit | |
| — | | |
| — | |
| Loss from Continuing Operations | |
| (10,796,137 | ) | |
| (16,984,696 | ) |
| Loss from discontinued operations, net of taxes | |
| (1,935,279 | ) | |
| (2,438,630 | ) |
| Net Loss | |
$ | (12,731,416 | ) | |
$ | (19,423,326 | ) |
| | |
| | | |
| | |
| Loss per share from continuing operations, basic and diluted | |
$ | (10.78 | ) | |
$ | (22.84 | ) |
| Loss per share from discontinued operations, basic and diluted | |
$ | (1.93 | ) | |
$ | (3.28 | ) |
| Net loss per share, basic and diluted | |
$ | (12.71 | ) | |
$ | (26.12 | ) |
| Weighted average shares, basic and diluted | |
| 1,001,505 | | |
| 743,550 | |
The accompanying notes are an integral part of
these consolidated financial statements.
ADIAL PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’
EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2022 AND 2021
| | |
Common Stock | | |
Additional
Paid In | | |
Accumulated | | |
Total Stockholders’ | |
| | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Equity | |
| Balance, December 31, 2020 | |
| 575,724 | | |
$ | 576 | | |
$ | 35,505,279 | | |
$ | (31,519,789 | ) | |
$ | 3,986,066 | |
| Stock-based compensation | |
| — | | |
| — | | |
| 2,259,517 | | |
| — | | |
| 2,259,517 | |
| Stock-based compensation, common stock issued for services | |
| 28,520 | | |
| 29 | | |
| 1,980,871 | | |
| — | | |
| 1,980,900 | |
| Options exercised | |
| 8,622 | | |
| 9 | | |
| 469,492 | | |
| — | | |
| 469,501 | |
| Warrant exercises | |
| 28,500 | | |
| 28 | | |
| 1,424,970 | | |
| — | | |
| 1,424,998 | |
| Stock issued in consideration of purchase of subsidiary | |
| 27,999 | | |
| 28 | | |
| 1,060,122 | | |
| — | | |
| 1,060,150 | |
| Sale of common stock | |
| 168,503 | | |
| 168 | | |
| 11,749,837 | | |
| — | | |
| 11,750,005 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| (19,423,326 | ) | |
| (19,423,326 | ) |
| Balance, December 31, 2021 | |
| 837,868 | | |
$ | 838 | | |
$ | 54,450,088 | | |
$ | (50,943,115 | ) | |
$ | 3,507,811 | |
| Stock-based compensation | |
| — | | |
| — | | |
| 2,101,210 | | |
| — | | |
| 2,101,210 | |
| Stock-based compensation, common stock issued for services | |
| 62,133 | | |
| 62 | | |
| 1,273,221 | | |
| — | | |
| 1,273,283 | |
| Sale of common stock | |
| 92,890 | | |
| 93 | | |
| 9,123,648 | | |
| — | | |
| 9,123,741 | |
| Warrant exercises | |
| 74,600 | | |
| 74 | | |
| 1,791 | | |
| — | | |
| 1,865 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| (12,731,416 | ) | |
| (12,731,416 | ) |
| Balance, December 31, 2022 | |
| 1,067,491 | | |
$ | 1,067 | | |
$ | 66,949,958 | | |
$ | (63,674,531 | ) | |
$ | 3,276,494 | |
The accompanying notes are an integral part of
these consolidated financial statements.
ADIAL PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | |
For the Years Ended
December 31, | |
| | |
2022 | | |
2021 | |
| CASH FLOWS FROM OPERATING ACTIVITIES: | |
| | |
| |
| Loss from operations | |
$ | (10,796,137 | ) | |
$ | (16,984,696 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | |
| Stock-based compensation | |
| 3,374,493 | | |
| 4,240,417 | |
| Amortization of intangible assets | |
| 564 | | |
| 565 | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Prepaid research and development | |
| 9,931 | | |
| 223,104 | |
| Prepaid expenses and other current assets | |
| 40,060 | | |
| 112,188 | |
| Accrued expenses | |
| (1,413,603 | ) | |
| 1,520,291 | |
| Accrued expenses, related party | |
| 175,000 | | |
| — | |
| Accounts payable and other current liabilities | |
| 15,826 | | |
| (378,675 | ) |
| Net cash used in operating activities – continuing operations | |
| (8,593,866 | ) | |
| (11,266,806 | ) |
| Net cash used in discontinued operations | |
| (2,592,119 | ) | |
| (682,623 | ) |
| Net cash used in operating activities | |
| (11,185,985 | ) | |
| (11,949,429 | ) |
| | |
| | | |
| | |
| CASH FLOWS FROM INVESTING ACTIVITIES: | |
| | | |
| | |
| Net cash from (used) in investing activities - continuing operations | |
| — | | |
| — | |
| Net cash used in to invest in discontinued operations | |
| — | | |
| (34,016 | ) |
| Net cash used in investing activities | |
| — | | |
| (34,016 | ) |
| | |
| | | |
| | |
| CASH FLOWS FROM FINANCING ACTIVITIES: | |
| | | |
| | |
| Net proceeds from equity offerings | |
| 9,123,741 | | |
| 11,750,005 | |
| Proceeds from warrant exercises | |
| 1,865 | | |
| 1,424,998 | |
| Proceeds of options exercises | |
| — | | |
| 469,501 | |
| Net cash provided by financing activities – continuing operations | |
| 9,125,606 | | |
| 13,644,504 | |
| Net cash provided by financing activities – discontinued operations | |
| — | | |
| — | |
| Net cash provided by financing activities | |
| 9,125,606 | | |
| 13,644,504 | |
| | |
| | | |
| | |
| NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | |
| (2,060,379 | ) | |
| 1,661,059 | |
| | |
| | | |
| | |
| CASH AND CASH EQUIVALENTS-BEGINNING OF YEAR | |
| 6,062,173 | | |
| 4,401,114 | |
| | |
| | | |
| | |
| CASH AND CASH EQUIVALENTS-END OF YEAR | |
$ | 4,001,794 | | |
$ | 6,062,173 | |
| | |
| | | |
| | |
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | |
| | | |
| | |
| Interest paid | |
$ | — | | |
$ | — | |
| Income taxes paid | |
$ | — | | |
$ | — | |
| Issuance of common stock for acquisition | |
$ | — | | |
$ | 1,060,150 | |
| Contingent consideration for acquisition | |
$ | — | | |
$ | 732,287 | |
The accompanying notes are an integral part of
these consolidated financial statements.
ADIAL PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1 — DESCRIPTION OF BUSINESS
Adial Pharmaceuticals, Inc.
(“Adial”) was converted from a limited liability company formed under the name Adial Pharmaceuticals, LLC, formed on November
23, 2010 in the Commonwealth of Virginia to a corporation and reincorporated in Delaware on October 1, 2017. Adial is presently engaged
in the development of medications for the treatment or prevention of addictions and related disorders.
Adial’s wholly owned
subsidiary, Purnovate, Inc., was acquired on January 26, 2021, having been formed as Purnovate, LLC in December of 2019. Purnovate is
a drug development company with a platform focused on developing drug candidates for non-opioid pain reduction and other diseases and
disorders potentially targeted with adenosine analogs that are selective, potent, stable, and soluble. These consolidated financial statements
include the accounts of both Adial and Purnovate for the year ended December 31, 2022 and 2021. On May 16, 2023, an option for the purchase
of the business of Purnovate was exercised and business of Purnovate sold effective June 30, 2023; as a result, the results of Purnovate
operations and assets and liabilities of Purnovate are now reported in the consolidated financial statements as discontinued operations.
In June of 2022, the Company
released data from its ONWARD™ Phase 3 pivotal trial of its lead compound AD04 (“AD04”) for the treatment of Alcohol
Use Disorder. Both the U.S. Food and Drug Administration (“FDA”) and the European Medicines Authority (“EMA”)
have indicated they will accept heavy-drinking-based endpoints as a basis for approval for the treatment of Alcohol Use Disorder rather
than the previously required abstinence-based endpoints. The Company has obtained meetings with the FDA and national medicines authorities
in Europe to determine the path toward approval. Key patents have been issued in the United States, the European Union, and other jurisdictions
for which the Company has exclusive license rights. The active ingredient in AD04 is ondansetron, a serotonin-3 antagonist. Due to its
mechanism of action, AD04 has the potential to be used for the treatment of other addictive disorders, such as Opioid Use Disorder, obesity,
smoking, and other drug addictions.
2 — GOING CONCERN AND OTHER UNCERTAINTIES
The consolidated financial
statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”),
which contemplate continuation of the Company as a going concern. The Company is in a development stage and has incurred losses each year
since inception and has experienced negative cash flows from operations in each year since. Based on the current development plans for
AD04 in both the U.S. and international markets and other operating requirements, the Company does not believe that the existing cash
and cash equivalents are sufficient to fund operations for the next twelve months following the filing of these consolidated financial
statements. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
Based on the recently announced
results of its ONWARD Phase 3 trial, the Company has scheduled meetings with the FDA and various European national authorities to discuss
the appropriate next steps towards the expeditious development of AD04 and to seek product approval. The results of these meetings may
materially change the Company’s expectations concerning the expected development cost of AD04. The Company has sold its Purnovate
programs to a company formed for that purpose, which has reduced the Company’s operating expenses and has provided approximately
$800 thousand in working capital through that third party’s payment of $450 thousand in exercise fees and partial payment of $350
thousand of a total $1.05 million in cost reimbursements owed, of which an estimated $700 thousand remain due. However, even with payment
of the remaining reimbursements, cash on hand will not be sufficient to fund the Company’s operation for more than twelve months
from the date of these financial statements, and the Company will require additional capital. There is not any certainty that the Company
will be able to access additional capital on acceptable terms, if at all, to continue operations after whatever funds are received from
the buyer are expended. If unable to access sufficient capital, the Company would be required to delay, scale back or eliminate some or
all of its research and development programs or delay its approach to regulators concerning AD04, which would likely have a material adverse
effect on the Company and its financial statements.
The Company’s continued
operations will depend on its ability to raise additional capital through various potential sources, such as equity and/or debt financings,
grant funding, strategic relationships, or out-licensing in order to complete its subsequent clinical trial requirements for AD04. Management
is actively pursuing financing and other strategic plans but can provide no assurances that such financing or other strategic plans will
be available on acceptable terms, or at all. Without additional funding, the Company would be required to delay, scale back or eliminate
some or all of its research and development programs, which would likely have a material adverse effect on the Company and its financial
statements.
Other Uncertainties
Generally, the industry in
which the Company operates subjects the Company to a number of other risks and uncertainties that can affect its operating results and
financial condition. Such factors include, but are not limited to: the timing, costs and results of clinical trials and other development
activities versus expectations; the ability to obtain regulatory approval to market product candidates; the ability to manufacture products
successfully; competition from products sold or being developed by other companies; the price of, and demand for, Company products once
approved; the ability to negotiate favorable licensing or other manufacturing and marketing agreements for its products.
With the results of the ONWARD
trial having been released and regulatory approaches underway, the risk of delays to the Company’s development programs from COVID-19
are reduced. However, the ongoing effects of the ongoing coronavirus pandemic, such as supply chain disruptions and post-stimulus inflation,
may increase non-trial costs such as insurance premiums, increase the demand for and cost of capital, increase loss of work time from
key personnel, and negatively impact our other key vendors and suppliers. The full extent to which the COVID-19 pandemic impacts the clinical
development of AD04 and the Company’s suppliers and other commercial partners, will depend on future developments that are still
highly uncertain and cannot be predicted with confidence at this time, all of which could have a material adverse effect on our business,
financial condition, and results of operations.
3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation and Principals of Consolidation
The accompanying consolidated
financial statements have been prepared in accordance with GAAP. The financial statements represent the consolidation of the Company and
its subsidiary in conformity with GAAP. All intercompany transactions have been eliminated in consolidation.
Reverse Stock Split
On August 4, 2023, the Company effected a reverse
stock split of its outstanding shares of common stock, trading on Nasdaq under the symbol ADIL, at a ratio of 1-for-25. As a result of
the reverse split, the Company had 1,197,630 shares of common stock outstanding immediately after effecting the reverse split. The shares
authorized for issue under the Company’s charter remained 50,000,000 common stock. All references to common stock, stock warrants
to purchase common stock, stock options to purchase common stock, share data, per share data and related information contained in these
financial statements have been retrospectively adjusted to reflect the effect of the Reverse Stock Split for all periods presented.
Use of Estimates
The preparation of these consolidated
financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of
assets and liabilities, and disclosure of contingent liabilities at the date of the financial statements, and the reported amounts of
revenues and expenses during the reporting period. Actual results could differ from those estimates.
Significant items subject
to such estimates and assumptions include the valuation of stock-based compensation, accruals associated with third party providers supporting
clinical trials, and income tax asset realization.
Basic and Diluted Loss per Share
Basic and diluted loss per
share are computed based on the weighted-average outstanding shares of common stock, which are all voting shares. Diluted net loss
per share is computed giving effect to all proportional shares of common stock, including stock options, restricted stock, and warrants
to the extent dilutive. Basic net loss per share was the same as diluted net loss per share for the years ended December 31, 2022 and
2021 as the inclusion of all potential common shares outstanding would have an anti-dilutive effect.
The total potentially dilutive
common shares that were excluded for the years ended December 31, 2022 and 2021 were as follows:
| | |
Potentially
Dilutive Common
Shares Outstanding
December 31, | |
| | |
2022 | | |
2021 | |
| Warrants to purchase common shares | |
| 483,834 | | |
| 316,719 | |
| Common Shares issuable on exercise of options | |
| 172,679 | | |
| 143,412 | |
| Restricted shares subject to repurchase | |
| 43,333 | | |
| – | |
| Total potentially dilutive Common Shares excluded | |
| 699,846 | | |
| 460,131 | |
Cash and Cash Equivalents
The Company considers all
highly liquid investments with original maturities of three months or less to be cash equivalents. At times, the Company’s cash
balances may exceed the current insured amounts under the Federal Deposit Insurance Corporation. At December 31, 2022, the Company did
not exceed FDIC insurance limits but held approximately $3.8 million in non-FDIC insured cash equivalent accounts. Included in cash equivalents
are money market investments with maturity dates less than ninety days and are carried at fair value. Unrealized gain or loss are included
in the interest income and are immaterial to the financial statements. At December 31, 2021, the Company did not exceed FDIC insurance
limits but held approximately $3.7 million in non-FDIC insured cash equivalent investments.
Fair Value Measurements
FASB ASC 820, Fair Value Measurement,
(“ASC 820”) defines fair value as the price that would be received to sell an asset or paid to transfer a liability (an exit
price) in an orderly transaction between market participants at the reporting date. The methodology establishes consistency and comparability
by providing a fair value hierarchy that prioritizes the inputs to valuation techniques into three broad levels, which are described below:
| |
● |
Level 1 inputs are quoted market prices in active markets for identical assets or liabilities (these are observable market inputs). |
| |
● |
Level 2 inputs are inputs other than quoted prices included within Level 1 that are observable for the asset or liability (includes quoted market prices for similar assets or identical or similar assets in markets in which there are few transactions, prices that are not current or prices that vary substantially). |
| |
● |
Level 3 inputs are unobservable inputs that reflect the entity’s own assumptions in pricing the asset or liability (used when little or no market data is available). |
The fair value of cash and
cash equivalents and accounts payable approximate their carrying value due to their short-term maturities.
Research and Development
Research and development costs
are charged to expense as incurred and include supplies and other direct trial expenses such as fees due to contract research organizations,
consultants which support the Company’s research and development endeavors, the acquisition of technology rights without an alternative
use, and compensation and benefits of clinical research and development personnel. Certain research and development costs, in particular
fees to contract research organizations (“CROs”), are structured with milestone payments due on the occurrence of certain
key events. Where such milestone payments are greater than those earned through the provision of such services, the Company recognizes
a prepaid asset which is recorded as expense; where fees earned are greater than milestone payments, an accrued expense liability is recorded
as expense.
Stock-Based Compensation
The Company measures the cost
of option awards based on the grant date fair value of the awards. That cost is recognized on a straight-line basis over the period during
which the awardee was required to provide service in exchange for the entire award. The fair value of options is calculated using the
Black-Scholes option pricing model, based on key assumptions such as the expected volatility of the Company’s common stock, the
risk-free rate of return, and expected term of the options. The Company’s estimates of these assumptions are primarily based on
historical data, peer company data, government data, and the judgment of management regarding future trends.
Common shares issued are valued
based on the fair value of the Company’s common shares as determined by the market closing price of a share of our common stock
on the date of the commitment to make the issuance.
Income Taxes
The Company accounts for income
taxes using the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable
to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis and
tax carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the
years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities
of a change in tax rates is recognized in income in the period that includes the enactment date.
A valuation allowance is established
to reduce net deferred tax assets to the amount expected to be realized. The Company recognizes the effect of income tax positions only
if those positions are more likely than not of being sustained. Changes in recognition and measurement are reflected in the period in
which the change in judgment occurs. Interest and penalties related to unrecognized tax benefits are included in income tax expense. The
Company has generally recorded a full valuation allowance for its tax carryforwards, reflecting the judgment of Company management that
they are more likely than not to expire unused.
4 — DISCONTINUED OPERATIONS
On January 26, 2021 the Company
acquired 100% of the equity interests of Purnovate, Inc. (“Purnovate”), pursuant to the Equity Purchase Agreement, dated December
7, 2020, as amended. Mr. Stilley, Adial’s then CEO, owned 28.73% of the membership interests in Purnovate and, therefore, the acquisition
of Purnovate was considered a related party transaction.
The total consideration paid, which included issuance of 27,999 shares
of common stock is set forth in the table below:
| Total consideration paid | |
| |
| Cash consideration | |
$ | 350,000 | |
| Stock consideration | |
| 1,060,150 | |
| Contingent consideration | |
| 732,287 | |
| Total | |
$ | 2,142,437 | |
The table below sets forth
the allocation of the fair value of the Purnovate Net Acquired Assets.
| Cash | |
$ | 380,589 | |
| Research and development supplies | |
| 1,548,397 | |
| Property and equipment | |
| 6,954 | |
| Lease right of use assets | |
| 294,294 | |
| In-process research and development | |
| 455,000 | |
| Total identifiable assets acquired | |
| 2,685,234 | |
| | |
| | |
| Accounts payable and accrued liabilities | |
| 910 | |
| Notes payable | |
| 350,000 | |
| Lease liability | |
| 294,294 | |
| Paycheck protection program loan | |
| 29,088 | |
| Deferred tax liability | |
| 117,476 | |
| Total liabilities assumed | |
| 791,768 | |
| | |
| | |
| Total identifiable net assets acquired | |
| 1,893,466 | |
| | |
| | |
| Goodwill | |
| 248,971 | |
| Total | |
$ | 2,142,437 | |
The Company utilized a relative fair value approach to allocate the
fair value of the research and development supplies.
The Company operated Purnovate
from the date of acquisition until May 16, 2023, when Adovate, LLC (“Adovate”) exercised an option agreement and the business
of Purnovate sold effective June 30, 2023, subsequent to these consolidated financial statements. The CEO, founder, and a major equity
holder of Adovate was Mr. Stilley, the former CEO of the Company and a former director, so the sale was considered a related party transaction.
Consideration paid by Adovate included $800,000 in cash exercise fees and reimbursement prepayments, Adovate equity valued at $1,727,897,
and estimated expense reimbursements due of $737,276. The Company derecognized $603,099 in net assets as a result of the sale, realizing
a gain of $2,662,074. As a result of this sale, all the assets and liabilities and the operating results of Purnovate have been reclassified
as discontinued operations.
On September 18, 2023, the Company and Adovate executed a final purchase
agreement (“FAA”), as required under the terms of the option agreement. The Company and Adovate agreed to a final specification
of the reimbursable expenses due of $1,050,000, which, less the $350,000 already paid, left $700,000 due and payable by Adovate, of which
$350,000 was due withing two days of FAA execution and the remaining $350,000 due no later than December 2, 2023. The Company recognized
a charge of $37,276 on execution of the FAA, reflecting the adjustment to the amount of the reimbursements receivable.
On September 20, 2023, Adovate
paid $350,000 in reimbursements, leaving $350,000 due.
Assets and liabilities included
within discontinued operations on the Consolidated Balance Sheets at December 31, 2022 and 2021 are as follows:
| | |
December 31,
2022 | | |
December 31,
2021 | |
| ASSETS | |
| | |
| |
| Current Assets: | |
| | |
| |
| Cash and cash equivalents | |
$ | — | | |
$ | — | |
| Prepaid research and development | |
| 428,700 | | |
| — | |
| Total Current Assets | |
| 428,700 | | |
| — | |
| | |
| | | |
| | |
| Fixed Assets, net | |
| 50,424 | | |
| 58,149 | |
| Acquired in-process research and development | |
| 455,000 | | |
| 455,000 | |
| Right-to-use Asset | |
| 193,997 | | |
| 246,209 | |
| Goodwill | |
| 248,971 | | |
| 248,971 | |
| Total non-current assets | |
| 948,392 | | |
| 1,008,329 | |
| Total Assets | |
$ | 1,377,092 | | |
$ | 1,008,329 | |
| | |
| | | |
| | |
| LIABILITIES | |
| | | |
| | |
| Current Liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 117,424 | | |
$ | 24,904 | |
| Accrued expenses | |
| 191,490 | | |
| — | |
| Lease liability, current | |
| 56,828 | | |
| 49,585 | |
| Total Current Liabilities | |
| 365,742 | | |
| 74,489 | |
| | |
| | | |
| | |
| Long-term Liabilities: | |
| | | |
| | |
| Contingent liabilities | |
| 492,000 | | |
| 1,014,000 | |
| Lease liability, non-current | |
| 150,547 | | |
| 207,375 | |
| Deferred tax liability | |
| 22,897 | | |
| 23,399 | |
| Total long-term liabilities | |
| 665,444 | | |
| 1,244,774 | |
| Total Liabilities | |
$ | 1,031,186 | | |
$ | 1,319,263 | |
Income from discontinued operations,
net of tax for the years ended December 31, 2022 and 2021 are as follows:
| | |
For the Year Ended | |
| | |
December 31, | |
| | |
2022 | | |
2021 | |
| Operating Expenses: | |
| | |
| |
| Research and development expenses | |
$ | 2,226,690 | | |
$ | 563,648 | |
| General and administrative expenses | |
| 230,962 | | |
| 168,037 | |
| Impairment Charges | |
| — | | |
| 1,548,397 | |
| Total Operating Expenses | |
| 2,457,652 | | |
| 2,280,082 | |
| | |
| | | |
| | |
| Loss From Operations | |
| (2,457,652 | ) | |
| (2,280,082 | ) |
| | |
| | | |
| | |
| Other Income (Expense) | |
| | | |
| | |
| Interest income (expense) | |
| (129 | ) | |
| — | |
| Change in value of contingent liability | |
| 522,000 | | |
| (281,713 | ) |
| Gain on forgiveness of loan | |
| | | |
| 29,088 | |
| Total other income (expense) | |
| 521,871 | | |
| (252,625 | ) |
| | |
| | | |
| | |
| Loss before provision for income taxes | |
| (1,935,781 | ) | |
| (2,532,707 | ) |
| Income tax benefit | |
| 502 | | |
| 94,077 | |
| | |
| | | |
| | |
| Loss from discontinued operations, net of tax | |
$ | (1,935,279 | ) | |
$ | (2,438,630 | ) |
5 — ACCRUED EXPENSES
Accrued expenses consist of the following:
| | |
December 31,
2022 | | |
December 31,
2021 | |
| Clinical research organization services and expenses | |
$ | 123,386 | | |
$ | 1,826,479 | |
| Employee compensation | |
| 761,509 | | |
| 520,795 | |
| Pre-clinical and manufacturing expenses | |
| 5,816 | | |
| – | |
| Legal and consulting services | |
| 72,616 | | |
| 29,656 | |
| Total accrued expenses | |
$ | 963,327 | | |
$ | 2,376,930 | |
6 — RELATED PARTY TRANSACTIONS
In January 2011, the Company
entered into an exclusive, worldwide license agreement with The University of Virginia Patent Foundation d/b/a the University of Virginia
Licensing and Ventures Group (the “UVA LVG”) for rights to make, use or sell licensed products in the United States based
upon patents and patent applications made and held by UVA LVG (the “UVA LVG License”). The Company is required to pay compensation
to the UVA LVG, as described Note 9. A certain percentage of these payments by the Company to the UVA LVG may then be distributed to the
Company’s former Chairman of the Board who currently serves as the Company’s Chief Medical Officer in his capacity as inventor
of the patents by the UVA LVG in accordance with their policies at the time.
On December 7, 2020, the Company
entered into an Equity Purchase Agreement with Purnovate, LLC to purchase all of the outstanding membership interests of Purnovate from
the members of Purnovate (the “Members”), such that after the acquisition, Purnovate became a wholly owned subsidiary of Adial.
The Company’s then Chief Executive Officer and board member, William B. Stilley, and another Adial board member, James W. Newman,
were, directly or indirectly, members of Purnovate. Messrs. Stilley and Newman agreed to sell their membership interests on the same terms
as the other Members, except that Mr. Stilley is subject to a two (2) year lock up with respect to the sale and transfer of the stock
consideration that he receives so long as his employment has not been terminated by the Company without cause prior to the end of such
period. Mr. Stilley owned approximately 28.7% of the membership interest of Purnovate and Mr. Newman controlled two entities that, together,
own less than 1% of the membership interests of Purnovate. As a result of the foregoing, the Company formed a Special Committee of independent
members of its Board of Directors to review and negotiate the acquisition terms.
On January 26, 2021 the acquisition
was consummated, and Messrs. Stilley and Newman and the other members of Purnovate sold all of their membership interests in Purnovate
to the Company (see Note 4).
On March 11, 2021, the Company
entered into Securities Purchase Agreements (the “SPAs”) with each of Bespoke, three entities controlled by James W. Newman,
Jr., a member of the Company’s Board of Directors (“Newman”), and Keystone Capital Partners, LLC (“Keystone”),
pursuant to which: (i) Bespoke agreed to purchase an aggregate of 13,466 shares of the Company’s common stock at a purchase price
of $75.00 per share for aggregate gross proceeds of $1,010,001; (ii) Newman agreed to purchase an aggregate of 1,200 shares of the Company’s
common stock at a purchase price of $75.00 per share for aggregate gross proceeds of $90,000; and (iii) Keystone agreed to purchase an
aggregate of 13,334 shares of the Company’s common stock at a purchase price of $75.00 per share for aggregate gross proceeds of
$1,000,002. In the year ended December 31, 2021, the Company issued 28,000 shares of common stock for total proceeds of $2,100,003. The
shares sold pursuant to the SPAs were registered though a registration statement on Form S-3 that was filed with the SEC on April 20,
2021 and declared effective on May 26, 2021.
On July 6, 2021, the Company
entered into Securities Purchase Agreements, dated July 6, 2021 (the “SPAs”), with three pre-existing investors for an aggregate
investment of $5,000,004 in consideration of the purchase by such investors of an aggregate of 66,666 shares of the Company’s common
stock at a purchase price of $75.00 per share. SPAs were entered with each of Bespoke Growth Partners, Inc. (“Bespoke”), a
company controlled by Mark Peikin, the Chief Strategy Officer of the Company at that time, Keystone Capital Partners, LLC (“Keystone”),
and Richard Gilliam, a private investor (“Gilliam”) (collectively, the “Investors,” and each an “Investor”),
pursuant to which: (i) Bespoke agreed to purchase an aggregate of 33,333 shares of the Company’s common stock at a purchase price
of $75.00 per share for aggregate gross proceeds of $2,500,002; (ii) Keystone agreed to purchase an aggregate of 20,000 shares of the
Company’s common stock at a purchase price of $75.00 per share for aggregate gross proceeds of $1,500,000; and (iii) Gilliam agreed
to purchase an aggregate of 13,333 shares of the Company’s common stock at a purchase price of $75.00 per share for gross proceeds
of $1,000,002.
Under the terms of the SPAs,
on July 7, 2021: (i) Bespoke purchased 3,333 shares of the Company’s common stock and agreed to purchase an additional 30,000 shares
of the Company’s common stock upon the effectiveness of the Registration Statement; (ii) Keystone purchased 2,000 shares of the
Company’s common stock and agreed to purchase an additional 18,000 shares of the Company’s common stock upon the effectiveness
of the Registration Statement; and (iii) Gilliam purchased 1,333 shares of the Company’s common stock and agreed to purchase an
additional 12,000 shares of the Company’s common stock upon the effectiveness of the Registration Statement.
Under the terms of the SPAs,
on August 2, 2021, Bespoke purchased 30,000 shares of the Company’s common stock for proceeds of $2,250,000; and on August 4, 2021,
Keystone purchased 18,000 shares of the Company’s common stock for proceeds of $1,350,000 and Gilliam purchased 12,000 shares of
the Company’s common stock for proceeds of $900,000. The shares sold pursuant to the SPAs were registered though a registration
statement on Form S-3 that was filed with the SEC on July 20, 2021 and declared effective on July 29, 2021.
On October 5, 2021, the Company
released 8,000 shares of its common stock and 6,000 warrants, expiring July 31, 2023 and exercisable at $156.25 per share, beneficially
owned by Dr. Bankole Johnson, the Company’s Chief Medical Officer, from the Lock-Up Agreement by and between the Company and Dr.
Johnson, dated December 12, 2019, as amended, and the related Pledge and Security Agreement, by and between the Company and Dr. Johnson,
dated December 12, 2019, to permit the sale of such shares and warrants to Bespoke Growth Partners, Inc. in a private transaction.
On November 9, 2021, the Company
entered into a Securities Purchase Agreement with Bespoke Growth Partners, Inc. Pursuant to the terms of the agreement, Bespoke agreed
to purchase up to 8,000 shares of common stock of the Company at a price of $100.00 per share for an aggregate investment of $800,000.
Bespoke has a pre-existing relationship with the Company and is controlled by Mark Peikin, the Company’s Chief Strategy Officer.
Pursuant to the terms of the stock purchase agreement, Bespoke purchased an initial 800 shares of the Company’s common stock on
November 9, 2021 and agreed to purchase an additional 7,200 shares of the Company’s common stock upon the effectiveness of a registration
statement. On December 17, 2021, after the effectiveness of a registration statement on form S-3, the additional 7,200 were sold.
See Note 9 for related party
vendor, consulting, and lease agreements. See Note 10 for a related party sale of business.
7 — SHAREHOLDERS’ EQUITY
Common Stock Issuances
On January 26, 2021, 26,799
unregistered shares of common stock were issued to the shareholders of Purnovate, Inc., including William B. Stilley, the Company’s
then CEO and entities controlled by James Newman, a Director, in consideration of purchase of Purnovate, Inc., at a total cost of $1,060,150
(See Note 4.)
On February 8, 2021, an option
to purchase 400 shares of common stock at an exercise price of $36.25 per share was exercised for total proceeds of $14,500.
On February 25, 2021, previously
registered warrants to purchase 28,500 shares at an exercise fee of $50.00 per share were exercised for a total of $1,425,000.
During the year ended December
31, 2021, the Company issued 65,836 shares of common stock under the Keystone equity purchase agreement for total proceeds of $3,850,000.
During the year ended December
31, 2021, the Company issued 102,667 shares of common stock for total proceeds of $7,900,007 under securities purchase agreements.
During the year ended December
31, 2021, the Company issued 28,520 shares of common stock to consultants for services rendered and to employees at a total cost of $1,980,900.
On February 10, 2022, the
Company, entered into a securities purchase agreement with an accredited institutional investor (“the Investor”) providing
for the issuance of (i) 92,890 shares of the Company’s common stock, par value $0.001, (ii) pre-funded warrants to purchase up to
74,600 shares of common stock with an exercise price of $0.025 per share, which Pre-Funded Warrants are to be issued in lieu of shares
of common stock to ensure that the Investor does not exceed certain beneficial ownership limitations, and (iii) warrants, with a term
of five years and six months from the date of issuance, to purchase an aggregate of up to 159,115 shares of common stock at an exercise
price of $63.00 per share. The Company received net proceeds from the offering of $9,123,741 after deducting fees due to the placement
agent and the Company’s transaction expenses. On June 8, 2022, all of the pre-funded warrants issued on February 10, 2022 were exercised
for proceeds of $1,865, resulting in issuance of 74,600 shares common stock.
During the year ended December
31, 2022, the Company issued 18,800 shares of common stock at a cost of $818,350 to consultants for services rendered.
During the year ended December
31, 2022, the Company issued employees 50,000 shares of common stock as bonuses, of which 43,333 remain outstanding. The first issuance
of 10,000 shares were subject to forfeiture for nominal consideration to Company on termination of the employee, the shares no longer
being liable to forfeiture (“vesting”) on the following schedule: 1/24 of 6,667 shares vesting on the date of issue and the
first of each of the next twenty-three subsequent months, and 3,333 shares vesting on the third anniversary of the date of issue. On December
30, 2022, the 6,666 shares vesting over 24 months were canceled by the recipient at the recipient’s will, at which time the Company
recognized the remaining $191,325 cost of the unvested shares cancelled. The second issuance of 40,000 shares are subject to forfeiture
on the same conditions, but on the following schedule: 1/6 of the issued shares vesting six months from date of issue, then 1/12 vesting
at the end of each subsequent three month period, the entire grant being vested three years from date of issue. On December 31, 2022,
none of the uncancelled shares were vested and 43,333 shares remained subject to repurchase.
2017 Equity Incentive Plan
On October 9, 2017, the Company
adopted the Adial Pharmaceuticals, Inc. 2017 Equity Incentive Plan (the “2017 Equity Incentive Plan”); which became effective
on July 31, 2018. Initially, the aggregate number of shares of our common stock that may be issued pursuant to stock awards under the
2017 Equity Incentive Plan was 70,000 shares. On October 13, 2022, by a vote of the shareholders, the number of shares issuable under
the 2017 Equity Incentive Plan was increased to 380,000. At December 31, 2022, the Company had issued 89,933 shares and had outstanding
167,090 options to purchase shares of our common stock under the 2017 Equity Incentive Plan, as well as 5,586 options to purchase shares
of common stock that were issued before the 2017 Equity Incentive Plan was adopted, leaving 122,977 available for issue.
Stock Options
The following table provides
the stock option activity for the years ended December, 2022 and 2021:
| | |
Total
Options
Outstanding | | |
Weighted
Average
Remaining
Term
(Years) | | |
Weighted
Average
Exercise
Price | | |
Weighted
Average
Fair Value
at Issue | |
| Outstanding December 31, 2020 | |
| 106,754 | | |
| 8.09 | | |
$ | 62.00 | | |
$ | 28.25 | |
| Issued | |
| 45,280 | | |
| | | |
| 61.75 | | |
| 59.25 | |
| Exercised | |
| (8,623 | ) | |
| | | |
| 54.50 | | |
| 22.25 | |
| Outstanding December 31, 2021 | |
| 143,411 | | |
| 7.80 | | |
$ | 66.00 | | |
$ | 50.50 | |
| Issued | |
| 29,265 | | |
| | | |
| 42.50 | | |
| 34.75 | |
| Exercised | |
| – | | |
| | | |
| | | |
| | |
| Outstanding December 31, 2022 | |
| 172,676 | | |
| 7.21 | | |
$ | 62.00 | | |
$ | 47.75 | |
| Outstanding December 31, 2022, vested and exercisable | |
| 129,511 | | |
| 6.79 | | |
$ | 64.75 | | |
$ | 49.00 | |
At December 31, 2022, the total intrinsic value
of the outstanding options was zero dollars.
The Company used the Black
Scholes valuation model to determine the fair value of the options issued, using the following key assumptions for the years ended December
31, 2022 and 2021:
| | |
| December 31,
2022 | | |
| December 31,
2021 | |
| Fair Value per Share | |
$ | 14.75-50.00 | | |
$ | 55.25-113.00 | |
| Expected Term | |
| 6.0 years | | |
| 1.00-5.75 years | |
| Expected Dividend | |
$ | — | | |
$ | — | |
| Expected Volatility | |
| 107.88-116.54% | | |
| 108.35-110.69% | |
| Risk free rate | |
| 1.89-3.26% | | |
| 0.07-1.26% | |
During the year ended December
31, 2022, 29,266 options to purchase shares of common stock were granted at a fair value of $1,017,095, an approximate weighted average
fair value of $34.75 per option, to be amortized over a service a weighted average period of 2.89 years. As of December 31, 2022, $1,939,438
in unrecognized compensation expense will be recognized over a remaining service period of 1.52 years.
The components of stock-based
compensation expense included in the Company’s Statements of Operations for the years ended December 31, 2022 and 2021 are as follows:
| | |
Year ended December 31, | |
| | |
2022 | | |
2021 | |
| Research and development options expense | |
| 230,627 | | |
| 304,421 | |
| Total research and development expenses | |
| 230,627 | | |
| 304,421 | |
| General and administrative options expense | |
| 1,870,583 | | |
| 1,955,096 | |
| Stock issued to consultants and employees | |
| 1,273,283 | | |
| 1,980,900 | |
| Total general and administrative expenses | |
| 3,143,866 | | |
| 3,935,996 | |
| Total stock-based compensation expense | |
$ | 3,374,493 | | |
$ | 4,240,417 | |
Stock Warrants
The following table provides
the activity in warrants for the respective periods.
| | |
Total Warrants | | |
Weighted
Average
Remaining
Term
(Years) | | |
Weighted Average Exercise Price | | |
Average
Intrinsic
Value | |
| Outstanding December 31, 2020 | |
| 345,985 | | |
| 3.46 | | |
$ | 115.00 | | |
| 0.50 | |
| Issued | |
| 2,125 | | |
| | | |
| 56.50 | | |
| | |
| Exercised | |
| (28,500 | ) | |
| | | |
$ | 50.00 | | |
| | |
| Outstanding December 31, 2021 | |
| 319,610 | | |
| 2.63 | | |
$ | 120.50 | | |
| 3.50 | |
| Issued | |
| 241,716 | | |
| | | |
| 43.50 | | |
| | |
| Exercised | |
| (74,600 | ) | |
| | | |
$ | 0.03 | | |
| | |
| Outstanding December 31, 2022 | |
| 486,726 | | |
| 3.04 | | |
$ | 100.75 | | |
| 0.25 | |
This table includes warrants
to purchase 13,794 shares of common stock issued to consultants, including the 8,000 issued during the year ended December 31, 2022, with
a total fair value of $263,195 at time of issue, calculated using the Black Scholes model assuming an underlying security values of $51.50,
volatility rate of 107.88% risk-free rate of 1.71%, and an expected term of 6.5 years. During the year ended December 31, 2022, the Company
recognized $305,230 in expense associated with these warrants with no additional expense remaining to be recognized.
During the years ended December
31, 2022 and 2021, 74,600 and 28,500 warrants to purchase shares of common stock were exercised for total proceeds of $1,865 and $1,424,998,
respectively.
8 — INCOME TAXES
A reconciliation of the statutory
Federal income tax rate and effective rate of the provision for income taxes is as follows:
| | |
Year ended | |
| | |
December 31,
2022 | | |
December 31,
2021 | |
| Federal statutory rate | |
| 21.00 | % | |
| 21.00 | % |
| Stock Options | |
| (4.85 | )% | |
| (2.79 | )% |
| Impairment charges | |
| (0.00 | )% | |
| (0.00 | )% |
| Other Permanent Items | |
| (0.41 | )% | |
| (0.37 | )% |
| State Taxes | |
| 3.93 | % | |
| 2.99 | % |
| Increase in VA | |
| (19.67 | )% | |
| (20.82 | )% |
| Other | |
| 0.00 | % | |
| 0.00 | % |
| Effective tax rate | |
| 0.00 | % | |
| 0.00 | % |
Tax expense (benefit) for
the year ended December 31, 2022 is shown on the table below:
| | |
| Current | | |
| Deferred | | |
| Total | |
| Federal | |
| — | | |
| — | | |
| — | |
| State and Local | |
| — | | |
| — | | |
| — | |
| Total | |
| — | | |
| — | | |
| — | |
Deferred income taxes reflect
the net tax effects of temporary differences between the carrying amounts of assets and liabilities recognized for financial reporting,
and the amounts recognized for income tax purposes. The significant components of deferred tax assets and liabilities as of December 31,
2022 and 2021, respectively, are as follows:
Deferred Tax Assets &
Liabilities (rounded)
| | |
Deferred Tax Asset | |
| | |
2022 | | |
2021 | |
| Net operating loss carry-forward | |
| 11,103,347 | | |
| 9,491,011 | |
| Accrued Expenses | |
| 157,869 | | |
| 104,426 | |
| Lease Liability | |
| — | | |
| — | |
| Restricted stock | |
| 15,982 | | |
| — | |
| Section 174 R&D | |
| 442,373 | | |
| — | |
| Less: valuation allowance | |
| (11,719,187 | ) | |
| (9,595,093 | ) |
| Total tax assets | |
$ | 384 | | |
$ | 344 | |
| Fixed Asset | |
| — | | |
| — | |
| Intangible assets | |
| (384 | ) | |
| (344 | ) |
| ROU Assets | |
| — | | |
| — | |
| Total deferred tax labilities | |
$ | (384 | ) | |
$ | (344 | ) |
| Net deferred tax asset (liability) | |
$ | — | | |
$ | — | |
Because substantially all
of the assets and liabilities whose carrying values resulted the net deferred tax liability are classified as assets and liabilities of
discontinued operations, the deferred tax liability has been classified as a long-term liability of discontinued operations in these consolidated
financial statements. (See Note 4.)
The Company has a net operating
loss carry-forward of $43.6 million for Federal and of $37.5 million state tax purposes at December 31, 2022, that is potentially available
to offset future taxable income. NOLS generated prior to 2018 will expire in 2037 and the 20- year carryover limitation was eliminated
for losses generated after January 1, 2018, giving the taxpayer the ability to carry forward losses indefinitely. However, NOL carry forward
arising after January 1, 2018, will now be limited to 80 percent of Taxable income.
In assessing the realizability
of the deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets
will not be realized. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income, net operating
loss carryback potential and tax planning strategies in making these assessments.
Based upon the above criteria,
the Company believes that it is more likely than not that the remaining net deferred tax assets will not be realized. Accordingly, the
Company has recorded a valuation allowance of $11.7 million against the net deferred tax asset that is not realizable.
Section 382 of the Internal
Revenue Code (“Section 382”) imposes limitations on a corporation’s ability to utilize net operating losses if it experiences
an “ownership change.” In general terms, an ownership change may result from transactions increasing the ownership of certain
stockholders in the stock of a corporation by more than 50 percentage points over a three-year period. Any unused annual limitation may
be carried over to later years, and the amount of the limitation may under certain circumstances be increased by the built-in gains in
assets held by us at the time of the change that are recognized in the five-year period after the change.
The company has not performed
a study to assess whether an ownership change for purposes of Section 382 has occurred, or whether there have been multiple ownership
change since the Company’s inception, due to the significant costs and complexities associated with such study. If the company has
experienced a change in control, as defined by Section 382, at any time since its public offering, utilization of net operating loss carryforwards
would be subject to an annual limitation under Section 382. Any limitation may result in expiration of a portion of the net operating
losses before utilization.
On August 16, 2022, the Inflation
Reduction Act of 2022 (the “IR Act”) was signed into federal law. The IR Act provides for, among other things, a new U.S.
federal 1% excise tax on certain repurchases of stock by publicly traded U.S. domestic corporations and certain U.S. domestic subsidiaries
of publicly traded foreign corporations occurring on or after January 1, 2023. The excise tax is imposed on the repurchasing corporation
itself, not its shareholders from which shares are repurchased. The amount of the excise tax is generally 1% of the fair market value
of the shares repurchased at the time of the repurchase. However, for purposes of calculating the excise tax, repurchasing corporations
are permitted to net the fair market value of certain new stock issuances against the fair market value of stock repurchases during the
same taxable year. In addition, certain exceptions apply to the excise tax. The U.S. Department of Treasury has been given authority to
provide regulations and other guidance to carry out and prevent the abuse or avoidance of the excise tax. The excise tax applies in cases
where the total value of the stock repurchased during the taxable year exceeds $1,000,000. The Company does not anticipate that the IR
Act will have an impact on the Company’s tax liability in the near term.
The Company files tax returns
as prescribed by the tax laws of the jurisdiction in which they operate. In the normal course of business, the Company is subject to examination
of Federal and state jurisdiction where applicable based on the statute of limitations that apply in each jurisdiction. As of December
31, 2022, open years related to the federal and state Jurisdictions are 2021, 2020, & 2019. The company has no open tax audits with
any taxing authority as of December 31, 2022.
The Company had no uncertain
tax positions at December 31, 2022.
9 — COMMITMENTS AND CONTINGENCIES
License with University of Virginia Patent
Foundation
In January 2011, the Company
entered into an exclusive, worldwide license agreement with the University of Virginia Patent Foundation, dba UVA Licensing and Ventures
Group (“UVA LVG”) for rights to make, use or sell licensed products in the United States based upon the ten separate patents
and patent applications made and held by UVA LVG.
As consideration for the rights
granted in the UVA LVG License, the Company is obligated to pay UVA LVG yearly license fees and milestone payments, as well as a royalty
based on net sales of products covered by the patent-related rights. More specifically, the Company paid UVA LVG a license issue fee and
is obligated to pay UVA LVG (i) annual minimum royalties of $40,000 commencing in 2017; (ii) a $20,000 milestone payments upon dosing
the first patient under a Phase 3 human clinical trial of a licensed product, $155,000 upon the earlier of the completion of a Phase 3
trial of a licensed product, partnering of a licensed product, or sale of the Company, $275,000 upon acceptance of an NDA by the FDA,
and $1,000,000 upon approval for sale of AD04 in the U.S., Europe or Japan; as well as (iii) royalties equal to a 2% and 1% of net sales
of licensed products in countries in which a valid patent exists or does not exist, respectively, with royalties paid quarterly. In the
event of a sublicense to a third party, the Company is obligated to pay royalties to UVA LVG equal to a percentage of what the Company
would have been required to pay to UVA LVG had it sold the products under sublicense ourselves. In addition, the Company is required to
pay to UVA LVG 15% of any sublicensing income. A certain percentage of these payments by the Company to the UVA LVG may then be distributed
to the Company’s former Chairman of the Board who currently serves as the Company’s Chief Medical Officer in his capacity
as inventor of the patents by the UVA LVG in accordance with their policies at the time.
The license agreement may
be terminated by UVA LVG upon sixty (60) days written notice if the Company breaches its obligations thereunder, including failing to
make any milestone, failure to make required payments, or the failure to exercise diligence to bring licensed products to market. In the
event of a termination, the Company will be obligated to pay all amounts that accrued prior to such termination. The Company is required
to use commercially reasonable efforts to achieve the goals of submitting a New Drug Application to the FDA for a licensed product by
December 31, 2024 and commencing commercialization of an FDA approved product by December 31, 2025. If the Company were to fail to use
commercially reasonable effort and fail to meet either goal, the licensor would have the right to terminate the license.
The term of the license continues
until the expiration, abandonment or invalidation of all licensed patents and patent applications, and following any such expiration,
abandonment or invalidation will continue in perpetuity on a royalty-free, fully paid basis.
During both the years ended
December 31, 2022 and 2021, the Company recognized $40,000 minimum license royalty expenses under this agreement. On May 13, 2022, the
Company acknowledged that its Phase 3 trial was complete according to the terms of the UVA LVG license and recognized an accrued license
royalty expense of $155,000, which payment was made on September 6, 2022. At December 31, 2022 and 2021, total accrued royalties and fees
due to UVA LVG were $40,000 and $0, respectively, shown on balance sheet as accrued expenses, related party.
Clinical Research Organization (CRO)
On October 31, 2018, the Company
entered into a master services agreement (“MSA”) with Crown CRO Oy (“Crown”) for contract clinical research and
consulting services. The MSA has a term of five years, automatically renewed for two-year periods, unless either party gives written notice
of a decision not to renew the agreement six months prior to automatic renewal. The MSA or a service agreement under it may be terminated
by the Company, without penalty, on fourteen days written notice for scientific, administrative, or financial reasons, or if the purpose
of the study becomes obsolete. In the event that the MSA or Service Order are terminated, Crown’s actual costs up the date of termination
will be payable by the Company, but any unrealized milestones would not be owed.
On November 16, 2018, the
Company and Crown entered into Service Agreement 1 under the MSA for a 24 week, multi-centered, randomized, double-blind, placebo-controlled,
parallel-group, Phase 3 clinical study of the Company’s lead compound, AD04 for fees, as amended, of $3,398,957 (€3,168,895
converted to dollars at the Euro/US Dollar exchange rate of 1.0726 as of December 31, 2022) milestone payments. On May 13, 2022, the Company
acknowledged the milestone event of the last patient having made the last clinical visit and made a payment of $146,765, and on June 30,
2022 the Company acknowledged the additional milestone event of the trial database being locked at which time it recognized a payable
of $137,375.
On April 28, 2022, the Company
and Crown settled a previous dispute concerning a putative change order. As part of this agreement, the Company agreed to pay Crown a
total of $454,034 (€410,000) for changes to the services described in Service Order 1. The settlement also altered the schedule of
remaining milestones to be as described in the table below.
At December 31, 2022, the
remaining future milestone payments are shown in the table below, converted to dollars from euros at the exchange rate then prevailing.
| Milestone Event | |
Percent
Milestone
Fees | | |
Amount | |
| eTMF Transfer | |
| 5 | % | |
$ | 144,768 | |
During the year ended December
31, 2022, the Company recognized $209,689 in non-cash income associated with the Service Agreement 1 and the settlement described above,
classified as a negative R&D expense. The negative expense was a result of the value of the settlement and total, fully earned value
of milestones being less than the expense previously accrued. On December 31, 2022 there remained an accrued R&D expense of $123,386
related to direct expenses under this agreement, which expense is expected to be fully paid with the occurrence of the final milestone
payment. (See Note 9 for the occurrence of the final milestone and recognition of this invoice.)
Service Agreement 1 also estimated
approximately $2.1 million (€2.2 million) in pass-through costs, mostly fees to clinical investigators and sites, which are billed
as incurred and the total contingent upon individual site rate and enrollment rates. With clinical enrollment having ended, the Company
has recorded approximately $3.5 million in site fees over the entire conduct of the trial, and does not expect to record material additional
site expenses.
Consulting Agreements – Related Party
On March 24, 2019, the Company
entered into a consulting agreement (the “Consulting Agreement”) with Dr. Bankole A. Johnson, who at the time of the
agreement was serving as the Chairman of the Board of Directors, for his service as Chief Medical Officer of the Company. The Consulting
Agreement has a term of three years, unless terminated by mutual consent or by the Company for cause. Dr. Johnson resigned as Chairman
of the Board of Directors at the time of execution of the consulting agreement. Under the terms of the Consulting Agreement, Dr. Johnson’s
annual fee of $375,000 per year is paid twice per month. On September 8, 2022, Dr. Johnson’s consulting agreement was amended to
increase his annual compensation to $430,000 annually and to pay him series of bonuses in cash and shares on the occurrence of certain
milestones. The Company recognized $416,200 and $375,000 in compensation expense in the years ended December 31, 2022 and 2021, respectively.
On July 5, 2019, the Company
entered into a Master Services Agreement (the “MSA”) and attached statement of work with Psychological Education Publishing
Company (“PEPCO”) to administer a behavioral therapy program during the Company’s upcoming Phase 3 clinical trial. PEPCO
is owned by a related party, Dr. Bankole Johnson. It is anticipated that the compensation to be paid to PEPCO for services under the MSA
will total approximately $300,000, of which shares of the Company’s common stock having a value equal to twenty percent (20%) of
this total can be issued to Dr. Johnson in lieu of cash payment.
As of December 31, 2022, the
Company had recognized all expenses associated with this agreement. No further expenses associated with the PEPCO MSA work order are expected.
Consulting Agreement – Related Party
On October 24, 2022, the Company
entered into a Master Services Agreement (the “MSA”) with Abuwala & Company, LLC, dba as Orbytel, for provision of strategic
consulting services. Orbytel made it known that it intended to utilize the services of the Keswick Group, LLC as a subcontractor in the
provision of these services. Tony Goodman, a director, is the founder and principal of Keswick Group, LLC, therefor Orbytel was considered
a related party. Statement of work #1 (“SOW #1”), executed with the MSA, committed the Company to $209,250 in payments. During
the year ended December 31, 2022, the Company recognized $151,500 in consulting expenses associated with this agreement, with $135,000
being recognized as related party accrued expenses on the balance sheet. The Company expected $57,750 in remaining expenses under SOW
#1 at December 31, 2022.
Other Consulting and Vendor Agreements
The Company has entered into
a number of agreements and work orders for future consulting, clinical trial support, and testing services, with terms ranging between
12 and 30 months. These agreements, in aggregate, commit the Company to approximately $430 thousand in future cash.
Litigation
The Company is subject, from
time to time, to claims by third parties under various legal disputes. The defense of such claims, or any adverse outcome relating to
any such claims, could have a material adverse effect on the Company’s liquidity, financial condition and cash flows. As of December
31, 2022, the Company did not have any pending legal actions.
10 — SUBSEQUENT EVENTS
Purnovate Sale
On January 27, 2023, the Company
and Adovate, LLC (then Adenomed, LLC) entered into an Option Agreement pursuant to which the Company granted to the Buyer an exclusive
option for a period of one hundred twenty (120) days from the effective date of the Agreement (the “Option Term”) for Buyer
or its designated affiliate to acquire all of the assets of Purnovate, the Company’s wholly owned subsidiary. The CEO, founder,
and a major equity holder of Adovate is a current director and the former CEO of the Company, so transactions with Adovate are considered
related party transactions.
As discussed in Note 4, on
May 8, 2023, Adovate sent a letter exercising its option effective May 16, 2023 for the purchase of the assets and business of Purnovate
and made payment of the $450,000 in fees due on exercise. Under the terms of the option agreement, on option exercise Adovate became liable
for reimbursement of all Purnovate operating expenses incurred and paid after December 1, 2022, such reimbursement to be paid within thirty
days of execution of the final acquisition agreement with the Company holding a security interest in the assets of Adovate until the expense
reimbursement is paid in full. The Company estimated that, at option exercise, the total reimbursement due was approximately $1,090,000.
On June 30, 2023, Adovate made a prepayment of $350,000 against the total reimbursement due, leaving approximately $740,000 in estimated
reimbursements due. On June 30, 2023, Adovate issued to the Company a 19.9% equity stake in Adovate as part of consideration owed, which
the Company valued at $1,727,897 and the Company completed the sale of the assets and business of Purnovate. Consideration paid by Adovate
also included contingent payments based on the occurrence of certain milestone events and a contingent royalty on future sales. No value
has been imputed to these contingent payments on the Company’s balance sheet, since it is at present less likely than not that such
payments will ever be made. Total consideration paid or receivable was $3,265,173. The Company derecognized $603,099 in net assets as
a result of the sale, realizing a gain of $2,662,074.
On September 18, 2023, the
Company and Adovate executed a final purchase agreement (“FAA”), as required under the terms of the option agreement. The
Company and Adovate agreed to a final specification of the reimbursable expenses due of $1,050,000, which, less the $350,000 already paid,
left $700,000 due and payable by Adovate, of which $350,000 was due withing two days of FAA execution and the remaining $350,000 due no
later than December 2, 2023. The Company recognized a charge of $37,276 on execution of the FAA, reflecting the adjustment to the value
of the reimbursements receivable.
On September 20, 2023, Adovate
paid $350,000 in reimbursements, leaving $350,000 due.
The assets, liabilities, and
results of operations of Purnovate, Inc. have been retroactively reclassified as discontinued for purposes of these financial statements.
Securities Purchase Agreement
On February 23, 2023, the
Company entered into a securities purchase agreement (the “2023 Purchase Agreement”) with an accredited institutional investor
(the “Investor”) providing for the issuance of 73,144 shares of the Common Stock, par value $0.001 (the “Common
Stock”). Pursuant to the 2023 Purchase Agreement, the Investor purchased the Shares for an aggregate purchase price of $750,000 with
net proceeds of $609,613, after placement agent fees and expenses. Pursuant to the Purchase Agreement, an aggregate of 73,144 Shares
were issued to the Investor. The Company issued to the Placement Agent a warrant (the “Placement Agent Warrants”) to purchase
up to an aggregate of 7,317 shares of common stock.
Master Services Agreement – Related Party
Effective March 15, 2023,
the Company entered in a master services agreement (the “Services Agreement”) with The Keswick Group, LLC, of which Tony Goodman,
a director of the Company, is the founder and principal, pursuant to which The Keswick Group, LLC has agreed to serve as a business consultant
to lead the Company’s partnering efforts for AD04 for nine months at a monthly fee of $22,000, with a performance bonus of 100,000
shares of the Company’s restricted common stock issuable upon the Company’s completion of a partnering agreement for AD04
through its efforts. The opportunity to earn the restricted stock performance bonus expires if a partnering agreement has not been completed
before the termination of the agreement. The Services Agreement may be terminated by either party upon thirty (30) days’ notice.
Options Issued
On May 23, 2023, the Company
issued options to purchase 39,800 shares of common stock with an exercise price of $7.50 per share, a term of ten years, and which vest
monthly pro rata over three years to officers, directors, and employees.
Standby Equity Purchase Agreement
On May 31, 2023, the Company
entered into an Equity Purchase Agreement with Alumni Capital, LLC (“Alumni”). This agreement constituted a standby equity
purchase agreement (a “SEPA”). Pursuant to the SEPA, the Company has the right, but not the obligation, to sell to Alumni
up to $3,000,000 of newly issued shares, subject to increase to $10,000,000 at the option of the Company, at the Company’s request
at any time during the commitment period, subject to certain conditions, which commenced on May 31, 2023 and will end on the earlier of
(i) December 31, 2024, or (ii) the date on which Alumni shall have made payment of advances requested by the Company totaling up to the
commitment amount of $3,000,000.
On August 4, 2023, the Company
sold 20,550 shares of common stock to Alumni under the terms of the SEPA for $6.83 per share, for total proceeds of $140,305.
31
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=ADIL_WarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Adial Pharmaceuticals (NASDAQ:ADIL)
Gráfico Histórico do Ativo
De Jan 2025 até Fev 2025
Adial Pharmaceuticals (NASDAQ:ADIL)
Gráfico Histórico do Ativo
De Fev 2024 até Fev 2025