HeartSciences Announces Closing of License Agreements with the Icahn School of Medicine at Mount Sinai to Develop and Commercialize AI Cardiovascular Algorithms
20 Novembro 2023 - 10:00AM

Heart Test Laboratories, Inc. d/b/a HeartSciences
(Nasdaq: HSCS; HSCSW) ("HeartSciences" or the "Company"),
an artificial intelligence (AI)-powered medical technology company
focused on transforming ECGs/EKGs to save lives through earlier
detection of heart disease, today announced the closing of its
license agreements with the Icahn School of Medicine at Mount Sinai
(Icahn Mount Sinai), in New York, NY, to develop and commercialize
electrocardiographic AI algorithms and assets. The licenses result
in Icahn Mount Sinai becoming a shareholder in the Company.
Andrew Simpson, Chief Executive Officer of
HeartSciences, said, "We continue to advance initiatives that
fundamentally transform and de-risk HeartSciences. The
closing of our licenses with Icahn Mount Sinai now propels us
forward with our cardiovascular disease AI-ECG development
programs. Combined with the recent creation of a new FDA
product classification for AI-ECG algorithms that we believe paves
the way for a more structured and quicker 510(k) process of our
technologies, we have clear focus on our target milestones," said
Andrew Simpson, CEO of HeartSciences. “We believe AI-based ECG
algorithms close the diagnostic gap to detect heart disease much
earlier and at a lower cost and we look forward to accelerating
commercialization and broadening the range of prospective solutions
that we will provide.”
Erik Lium, PhD, the Chief Commercial Innovation
Officer at the Mount Sinai Health System and President of Mount
Sinai Innovation Partners, said, "We look forward to working with
HeartSciences, a company that aims to bring new AI-powered ECG
capabilities to market in an effort to enable the early detection
of heart disease and, ultimately, improve the lives of
patients."
The licenses from Icahn Mount Sinai grant rights
to multiple patented and unpatented AI-based ECG/EKG algorithms and
technologies for the screening and diagnosis of cardiovascular
disease. The licenses provide HeartSciences with an industry
leading library of AI-based ECG algorithms. HeartSciences intends
to deliver these algorithms using a cloud-based, hardware agnostic
platform, allowing HeartSciences to accept ECGs from millions of
existing ECG devices around the world, including its MyoVista
device. The algorithms were developed using a database of millions
of ECG records, enabling researchers to develop a range of AI-based
ECG algorithms using state-of-the art data science methods.
The AI cardiovascular algorithms are based on
technology developed by Icahn Mount Sinai faculty and licensed by
Mount Sinai to HeartSciences. Mount Sinai and Icahn Mount Sinai
faculty, including Girish Nadkarni, MD, Irene and Dr. Arthur M.
Fishberg Professor of Medicine at Icahn Mount Sinai, System Chief
of the Division of Data-Driven and Digital Medicine (D3M),
Co-Director of the Mount Sinai Clinical Intelligence Center
(MSCIC), and Co-Director of The Charles Bronfman Institute for
Personalized Medicine; Akhil Vaid, MD, Instructor in the Division
of Data-Driven and Digital Medicine (D3M) at Icahn Mount Sinai;
Joshua Lampert, MD, Medical Director of Machine Learning for Mount
Sinai Fuster Heart Hospital; Vivek Reddy, MD, Director of Cardiac
Arrhythmia Services for The Mount Sinai Hospital and The Leona M.
and Harry B. Helmsley Charitable Trust Professor of Medicine in
Cardiac Electrophysiology at Icahn Mount Sinai; and Son Duong, MD,
Assistant Professor of Pediatrics (Pediatric Cardiology) at Icahn
Mount Sinai, have a financial interest in this technology and in
HeartSciences. The financial interest of Mount Sinai faculty is
pursuant to the Mount Sinai Intellectual Property Policy.
About HeartSciences
Heart Test Laboratories, Inc. d/b/a
HeartSciences is a medical technology company focused on applying
innovative AI-based technology to an ECG (also known as an EKG) to
expand and improve an ECG's clinical usefulness. Millions of ECGs
are performed every week and the Company's objective is to improve
healthcare by making an ECG a far more valuable cardiac screening
tool, particularly in frontline or point-of-care clinical settings.
HeartSciences' first product candidate for FDA clearance, the
MyoVista® wavECG™, or the MyoVista®, is a resting 12-lead ECG that
is also designed to provide diagnostic information related to
cardiac dysfunction which has traditionally only been available
through the use of cardiac imaging. The MyoVista® also provides
conventional ECG information in the same test. The business model,
which involves the use of the MyoVista® Device and consumables for
each test, is expected to be "razor-razorblade" as the electrodes
used with the MyoVista® are proprietary to HeartSciences, and new
electrodes are required for every test performed.
For more information, please
visit: https://www.heartsciences.com.
Twitter: @HeartSciences
Safe Harbor Statement
This announcement contains forward-looking
statements within the meaning of Section 27A of the Securities Act
of 1933, as amended, and Section 21E of the Securities Exchange Act
of 1934, as amended. These forward-looking statements are made
under the "safe harbor" provisions of the Private Securities
Litigation Reform Act of 1995 and are relating to the Company's
future financial and operating performance. All statements, other
than statements of historical facts, included herein are
"forward-looking statements" including, among other things,
statements about HeartSciences' beliefs and expectations. These
statements are based on current expectations, assumptions and
uncertainties involving judgments about, among other things, future
economic, competitive and market conditions and future business
decisions, all of which are difficult or impossible to predict
accurately and many of which are beyond the Company's control. The
expectations reflected in these forward-looking statements involve
significant assumptions, risks and uncertainties, and these
expectations may prove to be incorrect. Investors should not place
undue reliance on these forward-looking statements, which speak
only as of the date of this press release. Potential risks and
uncertainties include, but are not limited to, risks discussed in
HeartSciences' Annual Report on Form 10-K for the fiscal year ended
April 30, 2023, filed with the U.S. Securities and Exchange
Commission (the "SEC") on July 18, 2023, HeartSciences’ Quarterly
Report on Form 10-Q for the fiscal quarter ended July 31, 2023,
filed with the SEC on September 14, 2023, and in HeartSciences'
other filings with the SEC at www.sec.gov. Other than as required
under the securities laws, the Company does not assume a duty to
update these forward-looking statements.
For Investor and Media Inquiries, please
contact:
HeartSciences Gene Gephart +1-737-414-9213 (US)
info@heartsciences.com
Investors Gilmartin Group Vivian
Cervantes investorrelations@heartsciences.com
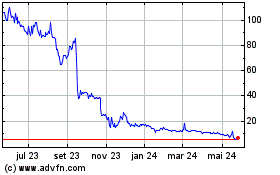
HeartSciences (NASDAQ:HSCS)
Gráfico Histórico do Ativo
De Dez 2024 até Jan 2025
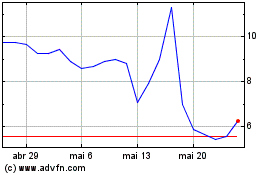
HeartSciences (NASDAQ:HSCS)
Gráfico Histórico do Ativo
De Jan 2024 até Jan 2025