Transgene to Present a Poster on Updated Data for TG4050 at SITC 2024
07 Outubro 2024 - 3:00AM

Transgene will present median 24-month follow up data from
patients enrolled in the Phase I trial evaluating the
individualized cancer vaccine TG4050 in the adjuvant treatment of
head and neck cancer
Strasbourg, France, October 7, 2024, 8 a.m.
CET – Transgene (Euronext Paris: TNG), a biotech company
that designs and develops virus-based immunotherapies for the
treatment of cancer, will present a poster highlighting median
24-month follow up from the ongoing randomized Phase I trial of its
individualized therapeutic cancer vaccine, TG4050 at the 39th
Society for ImmunoTherapy of Cancer (SITC) annual
meeting. This presentation will emphasize efficacy and
disease-free survival outcomes in patients with head and neck
cancers. SITC annual meeting will take place in Houston, Texas,
USA, from November 6 to 10, 2024.
The abstract will be available on the SITC
website on November 5, 2024, from 3 p.m. CET.
Poster details
Title: “Randomized phase I trial of
adjuvant individualized TG4050 vaccine in patients with locally
advanced resected HPV-negative head and neck squamous cell
carcinoma (HNSCC)”.
- Poster and abstract number: 650
- Date and Time: Thursday, November 7, 2024 at 4 p.m. CET
- Author : C. Le Tourneau
TG4050 is an individualized immunotherapy being
developed for solid tumors that is based on Transgene’s myvac®
technology and powered by NEC’s longstanding artificial
intelligence (AI) expertise. TG4050 is being evaluated in a
randomized multicenter Phase I/II clinical trial as a single agent
in the adjuvant treatment of HPV-negative head and neck cancers
(NCT04183166).
Transgene previously presented data in April
2024, that showed that all patients who received TG4050 remained
disease-free after a median follow-up of 18.6 months, comparing
favorably to the observational arm which saw 3 out of 16 patients
relapse during the same time period.
Transgene and NEC are continuing the joint
development of TG4050 in this indication with a Phase II extension
of the trial, which is currently enrolling patients.
***
About Transgene
Transgene (Euronext: TNG) is a biotechnology
company focused on designing and developing targeted
immunotherapies for the treatment of cancer. Transgene’s programs
utilize viral vector technology with the goal of indirectly or
directly killing cancer cells. The Company’s clinical-stage
programs consist of a portfolio of therapeutic vaccines and
oncolytic viruses: TG4050, the first individualized therapeutic
vaccine based on the myvac® platform, TG4001 for the treatment of
HPV-positive cancers, as well as BT-001 and TG6050, two oncolytic
viruses based on the Invir.IO® viral backbone. With Transgene’s
myvac® platform, therapeutic vaccination enters the field of
precision medicine with a novel immunotherapy that is fully
tailored to each individual. The myvac® approach allows the
generation of a virus-based immunotherapy that encodes
patient-specific mutations identified and selected by Artificial
Intelligence capabilities provided by its partner NEC. With its
proprietary platform Invir.IO®, Transgene is building on its viral
vector engineering expertise to design a new generation of
multifunctional oncolytic viruses. Additional information about
Transgene is available at: www.transgene.frFollow us on social
media: X (previously-Twitter): @TransgeneSA – LinkedIn:
@Transgene
Contacts
| Transgene
Contacts: |
Transgene Media
Contact: |
| Media: |
MEDiSTRAVA |
| Caroline
Tosch |
Sylvie
Berrebi/Frazer Hall |
| Corporate
Communications Manager |
+ 44 (0)
203 928 6900 |
|
+33 (0)3 68 33 27 38 |
transgene@medistrava.com |
|
communication@transgene.fr |
|
| |
|
| Lucie
Larguier |
|
| Chief Financial
Officer |
|
| Nadege
Bartoli |
|
| IR Analyst &
Financial Communications Officer |
|
|
+33 (0)3 88 27 91 03 /00 |
|
|
investorrelations@transgene.fr |
|
Disclaimer
This press release contains forward-looking
statements, which are subject to numerous risks and uncertainties,
which could cause actual results to differ materially from those
anticipated. The occurrence of any of these risks could have a
significant negative outcome for the Company’s activities,
perspectives, financial situation, results, regulatory authorities’
agreement with development phases, and development. The Company’s
ability to commercialize its products depends on but is not limited
to the following factors: positive pre-clinical data may not be
predictive of human clinical results, the success of clinical
studies, the ability to obtain financing and/or partnerships for
product manufacturing, development and commercialization, and
marketing approval by government regulatory authorities. For a
discussion of risks and uncertainties which could cause the
Company’s actual results, financial condition, performance or
achievements to differ from those contained in the forward-looking
statements, please refer to the Risk Factors (“Facteurs de Risque”)
section of the Universal Registration Document, available on the
AMF website (http://www.amf-france.org) or on Transgene’s website
(www.transgene.fr). Forward-looking statements speak only as of the
date on which they are made, and Transgene undertakes no obligation
to update these forward-looking statements, even if new information
becomes available in the future.
- 20240306_CurtainRaiserSITC_EN
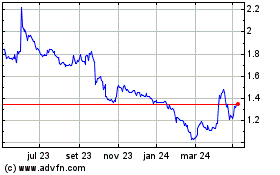
Transgene (EU:TNG)
Gráfico Histórico do Ativo
De Nov 2024 até Dez 2024
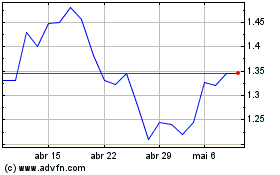
Transgene (EU:TNG)
Gráfico Histórico do Ativo
De Dez 2023 até Dez 2024