FDA Turns Away Eli Lilly Eczema Drug Due to Manufacturing Issue
02 Outubro 2023 - 8:25AM
Dow Jones News
By Colin Kellaher
The U.S. Food and Drug Administration has turned away Eli
Lilly's application seeking approval of lebrikizumab due to
findings that arose during a multi-sponsor inspection of a contract
manufacturing organization that included the monoclonal antibody
drug substance for the proposed eczema drug.
The Indianapolis drugmaker on Monday said the FDA issued a
so-called complete response letter, indicating that it wouldn't
approve the application in its current form.
Eli Lilly said the FDA didn't flag any concerns about the
clinical data package, safety or label for lebrikizumab, adding
that none of its other marketed or pipeline products are
affected.
Eli Lilly said it plans to work closely with the manufacturer
and the FDA to address the agency's concerns.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
October 02, 2023 07:10 ET (11:10 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
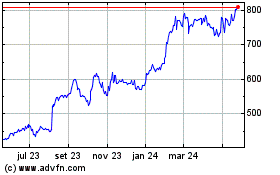
Eli Lilly (NYSE:LLY)
Gráfico Histórico do Ativo
De Nov 2024 até Dez 2024
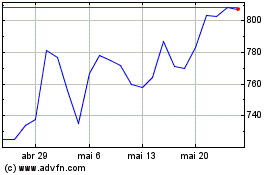
Eli Lilly (NYSE:LLY)
Gráfico Histórico do Ativo
De Dez 2023 até Dez 2024