- Based on topline results from the Phase 3
PHOENIX trial of AMX0035 in ALS, Amylyx has started a process with
the FDA and Health Canada of voluntarily discontinuing the
marketing authorizations for RELYVRIO/ALBRIOZA
- RELYVRIO/ALBRIOZA will no longer be available
for new patients as of today; Patients currently on therapy in the
U.S. and Canada who, in consultation with their physician, wish to
continue can be transitioned to a free drug program; PHOENIX Open
Label Extension is ongoing
- Amylyx continues to advance AMX0035 in
Wolfram syndrome and in progressive supranuclear palsy (PSP), and
AMX0114 in ALS
- Interim data from the Phase 2 HELIOS trial of
AMX0035 for the treatment of Wolfram syndrome are expected this
month and will be presented during a webcast on April 10, 2024
- Restructuring plan reduces workforce by
approximately 70% to focus resources on key clinical and
preclinical programs and extends expected cash runway into 2026,
through anticipated data readouts for AMX0035 in Wolfram syndrome
and PSP, and AMX0114 in ALS
Amylyx Pharmaceuticals, Inc. (NASDAQ: AMLX) (“Amylyx” or the
“Company”) today announced the Company has started a process with
the U.S. Food and Drug Administration (FDA) and Health Canada to
voluntarily discontinue the marketing authorizations for
RELYVRIO®/ALBRIOZA™ (sodium phenylbutyrate and taurursodiol [also
known as ursodoxicoltaurine]; also known as AMX0035) and remove the
product from the market in the U.S. and Canada based on topline
results from the Phase 3 PHOENIX trial. RELYVRIO/ALBRIOZA will no
longer be available for new patients as of today. Patients
currently on therapy in the U.S. and Canada who, in consultation
with their physician, wish to stay on treatment can be transitioned
to a free drug program.
“While this is a difficult moment for the ALS community, we
reached this path forward in partnership with the stakeholders who
will be impacted and in line with our steadfast commitment to
people living with ALS and other neurodegenerative diseases. The
decision to remove RELYVRIO/ALBRIOZA from the market and provide
therapy free of charge for those who wish to continue was informed
by the PHOENIX trial results, engagement with regulatory
authorities, and discussions with the ALS community. Thank you to
each and every person who shared feedback with us and continues to
support our commitment to the ALS community,” said Joshua Cohen and
Justin Klee, Co-CEOs of Amylyx.
Amylyx will continue to evaluate and share learnings from
PHOENIX to help inform future ALS research. At this time, Amylyx
intends to continue to collect available data on survival at the
encouragement of ALS specialists. The PHOENIX Open Label Extension
(OLE) is ongoing. Topline data from PHOENIX will be presented at
the American Academy of Neurology (AAN) Annual Meeting in Denver
and online, taking place April 13-18, 2024. The presentation is
scheduled to occur on April 16, 2024, during the Clinical Trials
Plenary Session (9:15 a.m. – 11:30 a.m. MT) and will be made
available on the “Publications and Presentations” section of the
Company’s website following the conclusion of the presentation.
As part of the Company’s purpose to discover and develop
innovative new treatment options for neurodegenerative diseases,
Amylyx continues to advance two key programs investigating its lead
asset AMX0035 in Wolfram syndrome and progressive supranuclear
palsy (PSP), and AMX0114, an antisense oligonucleotide targeting
calpain-2, in ALS.
“Our pipeline is supported by compelling clinical and
preclinical science demonstrating the potential of AMX0035 and
AMX0114 in neurodegenerative diseases. AMX0035 was designed to slow
or mitigate neurodegeneration by targeting endoplasmic reticulum
stress and mitochondrial dysfunction, two connected central
pathways that lead to cell death and neurodegeneration. We are
investigating AMX0035 in diseases where these two pathways are
implicated, which includes Wolfram syndrome and progressive
supranuclear palsy,” said Camille L. Bedrosian, MD, Chief Medical
Officer of Amylyx. “We look forward to presenting interim data from
our Phase 2 HELIOS study of AMX0035 in Wolfram syndrome, a rare,
genetic, fatal neurodegenerative disease with no FDA-approved
treatment options, later this month. In addition, our Phase 3 ORION
study remains ongoing to evaluate AMX0035 for the treatment of
progressive supranuclear palsy, a rare neurodegenerative disorder
characterized as a tauopathy. We continue to plan for an interim
analysis to evaluate the data from ORION that is now expected in
mid-2025.”
Dr. Bedrosian continued, “We also remain focused on ALS and
believe AMX0114 has strong potential for the treatment of ALS and
other diseases. Calpain-2 is considered an essential protein in the
process of axonal degeneration and has been repeatedly linked to
neurofilament biology in published studies. In our preclinical
studies of AMX0114 and in multiple independent published studies,
inhibition of calpain-2 has reduced cell death and degeneration and
decreased neurofilament levels. We expect to initiate a clinical
trial studying AMX0114 in ALS in the second half of this year.”
Amylyx also announced a restructuring to focus the Company’s
financial resources on upcoming clinical milestones. The Company
will reduce its workforce by approximately 70% and decrease
external financial commitments outside of its priority areas. With
these changes, Amylyx expects to have cash runway into 2026, which
will allow the organization to deliver on key upcoming milestones,
including data readouts from HELIOS (AMX0035 in Wolfram syndrome),
ORION (AMX0035 in PSP), and its planned trial of AMX0114 in
ALS.
“We are so thankful and grateful to our Amylyx team for their
contributions and steadfast dedication,” said Cohen and Klee.
“Together, the work we have accomplished across the world has
helped build a vital foundation to achieve our mission of one day
ending the suffering caused by neurodegenerative diseases, which
continue to have critical, unaddressed needs.”
HELIOS Interim Data in Wolfram Syndrome Investor Webcast
Details
Amylyx will host a virtual webcast with Dr. Fumihiko Urano,
Principal Investigator of the Phase 2 HELIOS clinical trial in
Wolfram syndrome and Professor Medicine in the Division of
Endocrinology, Metabolism & Lipid Research at Washington
University School of Medicine, to discuss interim HELIOS data on
April 10, 2024, at 1:30 p.m. ET. A live webcast of the presentation
can be accessed under “Events and Presentations” in the Investor
section of the Company’s website,
https://investors.amylyx.com/news-events/events, and will be
available for replay for 90 days following the event.
About AMX0035
AMX0035 is an oral, fixed-dose combination of sodium
phenylbutyrate (PB) and taurursodiol (TURSO; also known as
ursodoxicoltaurine outside of the U.S.). AMX0035 was designed to
slow or mitigate neurodegeneration by targeting endoplasmic
reticulum (ER) stress and mitochondrial dysfunction, two connected
central pathways that lead to cell death and neurodegeneration. We
believe that our proprietary combination of PB and TURSO and their
complementary mechanisms of action will allow us to synergistically
target abnormal cell death to better prevent neurodegeneration than
treatment targeted at either mechanism of action alone. AMX0035 is
being studied as a potential treatment for Wolfram syndrome and
progressive supranuclear palsy, two neurodegenerative diseases.
About AMX0114
AMX0114 is an antisense oligonucleotide designed to target the
gene encoding calpain-2, a key contributor to the axonal
(Wallerian) degeneration pathway. Axonal degeneration has been
recognized as an important early contributor to the clinical
presentation and pathogenesis of ALS and other neurodegenerative
diseases. Calpain-2 has been implicated in the pathogenesis of ALS
based on findings of elevated levels of calpain-2 and its cleavage
products in postmortem ALS tissue, therapeutic benefit of calpain-2
modulation in animal models of ALS, and the role of calpain-2 in
cleaving neurofilament, a broadly researched biomarker in ALS.
Preclinical studies completed to date have shown that AMX0114
achieves potent, dose-dependent, and durable knockdown of CAPN2
mRNA expression and calpain-2 protein levels in human motor
neurons. Moreover, in preclinical efficacy studies, treatment with
AMX0114 reduced extracellular neurofilament light chain levels
following neurotoxic insult in induced pluripotent stem cell
(iPSC)-derived human motor neurons, and improved survival of
iPSC-derived human motor neurons harboring ALS-linked, pathogenic
TDP-43 mutations.
About ALS
Amyotrophic lateral sclerosis (ALS, also known as motor neuron
disease) is a relentlessly progressive and fatal neurodegenerative
disorder caused by motor neuron death in the brain and spinal cord.
Motor neuron loss in ALS leads to deteriorating muscle function,
the inability to move and speak, respiratory paralysis, and
eventually, death. More than 90% of people with ALS have sporadic
disease, showing no clear family history. ALS affects around 30,000
people in the U.S., and more than 30,000 people are estimated to be
living with ALS in Europe (European Union and the United Kingdom).
People living with ALS have a median survival of approximately two
years from diagnosis.
About the HELIOS Trial
The HELIOS trial (NCT05676034) is a 12-participant, open-label,
proof of biology, Phase 2 trial designed to study the effect of
AMX0035 on safety and tolerability, and various measures of
endocrinological, neurological, and ophthalmologic function in
adult participants living with Wolfram syndrome.
About Wolfram Syndrome
Wolfram syndrome is an autosomal recessive neurodegenerative
disease characterized by childhood-onset diabetes, optic nerve
atrophy, and neurodegeneration. Common manifestations of Wolfram
syndrome include diabetes mellitus, optic nerve atrophy, central
diabetes insipidus, sensorineural deafness, neurogenic bladder, and
progressive neurologic difficulties. Genetic and experimental
evidence suggests that endoplasmic reticulum (ER) dysfunction is a
critical pathogenic component of Wolfram syndrome. The prognosis of
Wolfram syndrome is poor, and many people with the disease die
prematurely with severe neurological disabilities.
About the ORION Trial
The ORION trial (NCT06122662) is a global, randomized,
double-blind, placebo-controlled Phase 3 clinical trial designed to
assess the efficacy, safety, and tolerability of AMX0035 compared
to placebo in people living with progressive supranuclear palsy
(PSP). The ORION Phase 3 trial was designed and planned in
collaboration with key global academic leaders, people living with
PSP and their caregivers, and industry advocacy organizations.
About PSP
Progressive supranuclear palsy (PSP) is a sporadic, rare, and
adult-onset neurodegenerative disorder that affects walking and
balance, eye movement, swallowing, and speech. People living with
PSP have a life expectancy of six to eight years after initial
diagnosis, and its epidemiology is similar to that of amyotrophic
lateral sclerosis (ALS). PSP typically begins in late-middle age
and rapidly progresses over time. The disease affects approximately
seven in 100,000 people worldwide, and there are currently no
disease-modifying therapies approved for the treatment of PSP.
PSP is characterized by abnormal tau inclusions and is
consequently also known as a tauopathy. Similar to other
neurodegenerative diseases, pathophysiologic changes underlying PSP
are multifactorial, with several genetic and environmental factors
likely contributing to tau dysfunction and aggregation.
Multiple pathways, including genetic mutations, endoplasmic
reticulum (ER) stress, and the activation of unfolded protein
response, mitochondrial dysfunction, and neuroinflammation have
been implicated as contributors to tau dysfunction and
aggregation.
About Amylyx Pharmaceuticals
Amylyx Pharmaceuticals, Inc. is committed to supporting and
creating more moments for the neurodegenerative disease community
through the discovery and development of innovative new treatments.
Amylyx is headquartered in Cambridge, Massachusetts. For more
information, visit amylyx.com and follow us on LinkedIn and X,
(formerly Twitter). For investors, please visit
investors.amylyx.com.
Forward-Looking Statements
Statements contained in this press release regarding matters
that are not historical facts are “forward-looking statements”
within the meaning of the Private Securities Litigation Reform Act
of 1995, as amended. Because such statements are subject to risks
and uncertainties, actual results may differ materially from those
expressed or implied by such forward-looking statements. Such
statements include, but are not limited to, Amylyx’ expectations
regarding: interactions with regulatory authorities; the
availability of RELYVRIO/ALBRIOZA for patients currently taking
RELYVRIO/ALBRIOZA; the timing and significance of learnings from
the PHOENIX trial, the PHOENIX survival analysis and the Open
Label Extension; the continued evaluation of AMX0035 in other
neurodegenerative diseases, including the timing for expected data
readouts in the ORION and HELIOS studies; the potential for AMX0114
as a treatment for ALS and the planned initiation of a trial
evaluating AMX0114 in ALS; and the impact of resource-conserving
measures on extending the Company's cash runway, including the
restructuring plan. Any forward-looking statements in this press
release are based on management’s current expectations of future
events and are subject to a number of risks and uncertainties that
could cause actual results to differ materially and adversely from
those set forth in or implied by such forward-looking statements.
Risks that contribute to the uncertain nature of the
forward-looking statements include the risks and uncertainties set
forth in Amylyx’ United States Securities and Exchange Commission
(SEC) filings, including Amylyx’ Annual Report on Form 10-K for the
year ended December 31, 2023, and subsequent filings with the SEC.
All forward-looking statements contained in this press release
speak only as of the date on which they were made. Amylyx
undertakes no obligation to update such statements to reflect
events that occur or circumstances that exist after the date on
which they were made.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240404501040/en/
Media Amylyx Media Team (857) 799-7274
amylyxmediateam@amylyx.com Investors Lindsey Allen (857)
320-6244 Investors@amylyx.com
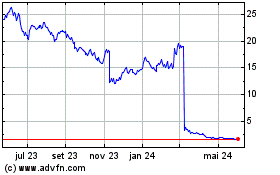
Amylyx Pharmaceuticals (NASDAQ:AMLX)
Gráfico Histórico do Ativo
De Dez 2024 até Jan 2025
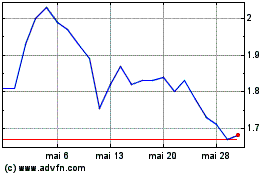
Amylyx Pharmaceuticals (NASDAQ:AMLX)
Gráfico Histórico do Ativo
De Jan 2024 até Jan 2025