Evolus Announces EU Approval of Estyme® Injectable Hyaluronic Acid Gels Under New Medical Device Regulation
31 Outubro 2024 - 3:02PM
Business Wire
Evolus, Inc. (NASDAQ: EOLS), a performance beauty company with a
focus on building an aesthetic portfolio of consumer brands, today
announced the EU Medical Device Regulation (MDR) certification was
received for four unique injectable hyaluronic acid (HA) gels under
the brand name Estyme® (pronounced “esteem”), branded as Evolysse™
in the U.S. market. This certification marks a key regulatory
milestone for Evolus, enabling it to commercially enter the dermal
filler segment and doubling its addressable market outside the U.S.
to $1.8 billion1.
The CE Mark certification, through the new MDR process
represents more stringent requirements, designed to ensure the
safety, efficacy, and quality of medical devices sold in Europe.
Achieving this certification demonstrates that the Estyme®
injectable HA gels meet the highest standards for product design,
production, and performance. Evolus will introduce Estyme® through
a limited experience program with select physician partners. The
limited experience program will allow Evolus to collaborate with
leading aesthetic practitioners across Europe, gathering valuable
insights to refine its broader launch strategy. A broader European
launch is planned for the second half of 2025.
“The MDR CE Mark certification for Estyme®, is a critical
milestone in our strategic path to expand our product portfolio and
reflects our commitment to delivering high-quality gels that adhere
to the most rigorous regulatory standards,” said David Moatazedi,
President and Chief Executive Officer. “With Estyme®, we are
bringing a new level of excellence to the dermal filler category,
as we grow our product portfolio to meet growing consumer demand
for premium, cash-pay aesthetic treatments.”
Dr. Rui Avelar, Chief Medical Officer and Head of R&D,
added, “We are proud that Estyme® has achieved CE Mark
certification under the MDR’s stringent standards, which reflects a
commitment to the highest standards of patient safety and product
efficacy. Estyme® is a differentiated line of injectable HA gels
currently being studied globally and the first innovation
breakthrough, with proprietary cold technology, in a decade. These
gels utilize breakthrough cross-linking technology that better
preserves the natural HA chain, which we believe will help
distinguish it from other products in this category. Designed with
the highest level of precision to meet the needs of both
practitioners and patients, we look forward to seeing its positive
impact on the European aesthetic market.”
The company remains on track for the U.S. approval and launch of
the Evolysse™ injectable HA gels beginning in 2025.
About Evolus, Inc.
Evolus (NASDAQ: EOLS) is a global performance beauty company
evolving the aesthetic neurotoxin market for the next generation of
beauty consumers through its unique, customer-centric business
model and innovative digital platform. Our mission is to become a
global, multi-product aesthetics company based on our flagship
product, Jeuveau® (prabotulinumtoxinA-xvfs), the first and only
neurotoxin dedicated exclusively to aesthetics and manufactured in
a state-of-the-art facility using Hi-Pure™ technology. Evolus is
expanding its product portfolio having entered into a definitive
agreement to be the exclusive U.S. distributor of Evolysse™, and
the exclusive distributor in Europe of Estyme®, a line of unique
injectable hyaluronic acid (HA) gels. These injectable HA gels are
currently in the late stages of the regulatory approval process,
with plans, upon approval, for a launch starting in 2025. Visit us
at www.evolus.com, and follow us on LinkedIn, X, Instagram or
Facebook.
Jeuveau® and Nuceiva®, are registered trademarks and Evolysse™
is a trademark of Evolus, Inc. Hi-Pure™ is a trademark of Daewoong
Pharmaceutical Co, Ltd. Estyme® is a trademark of Symatese
Aesthetics S.A.S.
1 Source: Medical Insights Dermal Filler Market Study, March
2023 (www.miinews.com) and Aesthetic Injectables | Market Insights
| Europe 2024 © 2023 Clarivate
View source
version on businesswire.com: https://www.businesswire.com/news/home/20241031746242/en/
Investors: Nareg Sagherian, Vice
President, Head of Global Investor Relations and Corporate
Communications Phone: (248) 202-9267 Email: ir@evolus.com
Media: Email: media@evolus.com
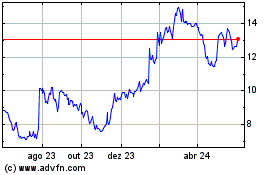
Evolus (NASDAQ:EOLS)
Gráfico Histórico do Ativo
De Nov 2024 até Dez 2024
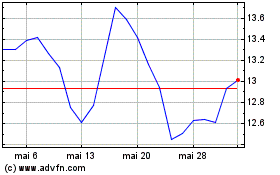
Evolus (NASDAQ:EOLS)
Gráfico Histórico do Ativo
De Dez 2023 até Dez 2024