Data Will be Presented in Two Posters at the
EASL Congress 2024
CAMBRIDGE,
Mass., May 22, 2024 /PRNewswire/ -- NeuroBo
Pharmaceuticals, Inc. (Nasdaq: NRBO), a clinical-stage
biotechnology company focused on the transformation of
cardiometabolic diseases, today announced that pre-clinical data
suggests that DA-1241, a novel G-Protein-Coupled Receptor 119
(GPR119) agonist, in combination with semaglutide (Wegovy®),
improves liver fibrosis and demonstrates additive hepatoprotective
effects in pre-clinical metabolic dysfunction-associated
steatohepatitis (MASH) models compared to either treatment alone.
Members of the Dong-A ST Research Center and Contract Research
Organization, Gubra, will present the data in two poster
presentations at the EASL Congress 2024, taking place June 5-8, in Milan,
Italy, as well as virtually.
"The data being presented at EASL further
strengthen the pre-clinical evidence that DA-1241's activation of
GPR119 has therapeutic potential for the reduction of hepatic
steatosis, inflammation, fibrosis, and improved glucose control,"
stated Hyung Heon Kim, President and
Chief Executive Officer of NeuroBo. "In April, we completed
enrollment for Part 1 of our Phase 2a clinical trial of DA-1241 in
MASH and continue to enroll patients in Part 2, exploring the
efficacy of DA-1241 in combination with sitagliptin, a DPP-4
inhibitor, which we believe will show synergistic effects compared
to DA-1241, alone. The new data being presented at the EASL
Congress explored DA-1241 in combination with semaglutide, a GLP1R
agonist. Importantly, the data suggests, for the first time, a
beneficial combination effect of DA-1241 and semaglutide in the
treatment of liver fibrosis, which may be attributed to augmented
inhibition of fibrogenesis and inflammation in the liver. The data
also demonstrates more than additive effects on metabolic,
biochemical, and histological endpoints in GAN DIO-MASH mice,
highlighting the therapeutic potential of dual targeting GPR119 and
GLP1R function in MASH with liver fibrosis. We eagerly anticipate
reporting top-line data from both parts of the ongoing Phase 2a
clinical trial in MASH in the fourth quarter of this year. We
continue to believe the unique mechanism of DA-1241, targeting the
inflammation associated with MASH, will translate into a safe and
effective treatment for this disease."
- Abstract Title: Additive Hepatoprotective Effects of
DA-1241, a Novel GPR119 Agonist, in Combination with Semaglutide in
the GAN Diet-Induced Obese and Biopsy-Confirmed Mouse Model of
MASH
- Authors: Monika Lewinska, Malte H. Nielsen, Susanne Pors, Henrik B.
Hansen, Il Hoon Jung, Hyung Heon
Kim, Michael Feigh,
Mi-Kyung Kim
- Presenter Name: Dr. Michael
Feigh, Vice President Scientific Research, Gubra
- Abstract Number: 1950
- Abstract ID: THU-232
- Session: Poster - MASLD: Experimental and
pathophysiology
- Session Date: Thursday, June 6,
2024
- Session Time: 8:30 am –
6:00 pm CET
Male C57BL/6JRj mice were fed the GAN diet for 36
weeks before treatment initiation. Only biopsy-confirmed GAN
DIO-MASH mice (steatosis score =3, lobular inflammation score ≥2,
fibrosis stage F2-F3) were stratified to treatment (n=14-15 per
group). GAN DIO-MASH mice received once daily treatment with
vehicle, DA-1241 (100 mg/kg, PO) or semaglutide (30 nmol/kg, SC)
alone or in combination for 8 weeks. Within-subject comparisons
(pre vs. post treatment) were performed for liver biopsy
histopathological NAFLD Activity Score (NAS) and Fibrosis Stage.
Terminal quantitative endpoints included plasma/liver biochemistry,
liver histomorphometry and RNA sequencing. DA-1241 was
weight-neutral and did not influence liver weight. In contrast,
semaglutide robustly reduced body weight by approximately 25% and
improved hepatomegaly in GAN DIO-MASH mice with or without
combination treatment. There was no additional weight loss in the
combination group compared to semaglutide alone. Each monotherapy
ameliorated plasma transaminases and liver cholesterol levels, with
combination therapy providing further improvement compared to
monotherapies.
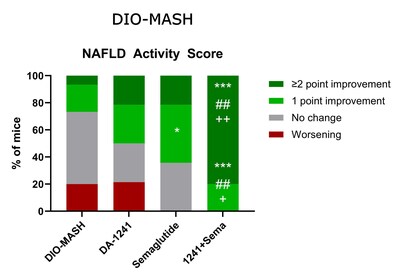
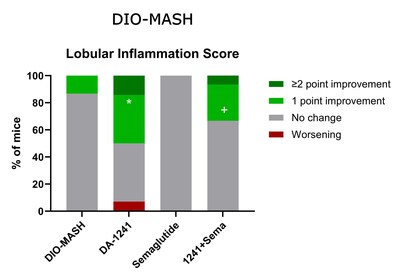
DA-1241 and semaglutide monotherapy each
improved NAS (≥2-point) in 21% of mice, whereas combination
treatment led to marked improvements (≥2-point in 80% of mice and
≥1-point in all mice), driven by reduction in steatosis and lobular
inflammation scores. Correspondingly, combination therapy
synergistically promoted quantitative histology for steatosis
(%-area of liver lipids, % lipid-laden hepatocytes) compared to
monotherapies. While treatments did not significantly influence
quantitative markers of fibrosis (PSR, Col1a1), DA-1241 and
semaglutide in monotherapy lowered α-SMA levels with further
improvement in combination treatment, suggesting additive
inhibitory effects on fibrogenesis. Liver transcriptome analysis
demonstrated a significant increase in the number of differential
expressed genes with prominent signature changes in lipid
metabolism, chemokine signaling, and fiber proteins following
combination therapy compared to monotherapies.
- Abstract Title: DA-1241, a GPR119 Agonist, Combined
with Semaglutide Synergistically Improved Liver Fibrosis in Mice
with CCl4 Induced Liver Fibrosis
- Authors: Il Hoon Jung, Tae
Hyoung Kim, Sujin Lee,
Yuna Chae, Hyung Heon Kim, Mi-Kyung
Kim
- Presenter Name: II Hoon
Jung, Dong-A ST Research Center
- Abstract Number: 117
- Abstract ID: THU-531
- Session: Poster - Fibrosis / Stellate cell biology
- Session Date: Thursday, June 6,
2024
- Session Time: 8:30 am –
6:00 pm CET
Liver fibrosis mice were generated by feeding a
western diet in adjunctive with CCl4 injections. After
administering CCl4 twice weekly for 3 weeks, mice with
elevated plasma ALT levels were allocated to receive DA-1241 (oral)
or semaglutide (subcutaneous) alone and in combination for 4 weeks.
At the end of treatment, semaglutide reduced body weight by
approximately 17% (p < 0.05 vs. vehicle control), while DA-1241
(-2.8%) showed little effect. There was no additional weight loss
in the combination group (-19%, p < 0.05) compared to
semaglutide alone. After four-week-treatment, three drug-treated
groups had significantly lower plasma ALT levels than the vehicle
control group, suggesting alleviation of liver damage.
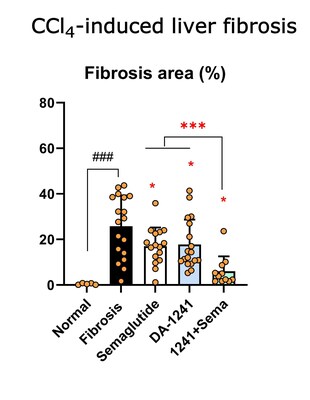
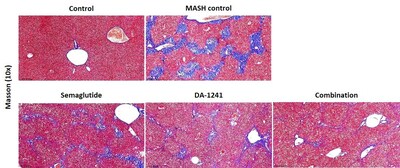
Collagen fiber deposition was prominent in mice
treated with vehicle compared to the normal control group. However,
DA-1241 or semaglutide alone lowered collagen-positive area
compared to the vehicle-treated group (17.8%, 17.1% vs. 25.8%, p
< 0.05), and their combination therapy elicited a further
reduction to 6.05% (p < 0.05) compared with each treatment
alone, which was recapitulated in changes of fibrosis score.
Hepatic hydroxyproline contents and gene expression of various
collagen subtypes (Col1a1, Col3a1, Col5a1, Col6a1) were also
altered correspondingly, supporting the beneficial combination
effects against the liver fibrogenesis. Notably, gene expression of
Hedgehog-interacting protein (Hhip), a suppressor of hepatic
stellate cell activation, was lower in mice with liver fibrosis
than in normal mice, and were increased by DA-1241 or semaglutide
alone. Intriguingly, their combination therapy fully restored the
gene expression of Hhip. Additionally, the expression of
inflammatory cytokines (Tnfa, IL1b, Ccl2, Cxcl10) was
significantly reduced by each monotherapy, and combination
treatment reduced gene expression of Tnfa and Cxcl10 more than
monotherapy. These data indicate that liver inflammation status has
improved as well.
After the presentations, the posters will be
accessible within the "Presentations" section of NeuroBo's website
at: https://www.neurobopharma.com/events-presentations/presentations.
About DA-1241
DA-1241 is a novel
G-Protein-Coupled Receptor 119 (GPR119) agonist with development
optionality as a standalone and/or combination therapy for both
MASH and type 2 diabetes (T2D). Agonism of GPR119 in the gut
promotes the release of key gut peptides GLP-1, GIP, and PYY. These
peptides play a further role in glucose metabolism, lipid
metabolism and weight loss. DA-1241 has beneficial effects on
glucose, lipid profile and liver inflammation, supported by
potential efficacy demonstrated during in vivo preclinical studies.
The therapeutic potential of DA-1241 has been demonstrated in
multiple pre-clinical animal models of MASH and T2D where DA-1241
reduced hepatic steatosis, inflammation, fibrosis, and improved
glucose control. Furthermore, in Phase 1a and 1b trials, DA-1241 was well tolerated in both
healthy volunteers and those with T2DM.
About NeuroBo Pharmaceuticals
NeuroBo
Pharmaceuticals, Inc. is a clinical-stage biotechnology company
focused on transforming cardiometabolic diseases. The company is
currently developing DA-1241 for the treatment of Metabolic
Dysfunction-Associated Steatohepatitis (MASH) and is developing
DA-1726 for the treatment of obesity. DA-1241 is a novel
G-protein-coupled receptor 119 (GPR119) agonist that promotes the
release of key gut peptides GLP-1, GIP, and PYY. In preclinical
studies, DA-1241 demonstrated a positive effect on liver
inflammation, lipid metabolism, weight loss, and glucose
metabolism, reducing hepatic steatosis, hepatic inflammation, and
liver fibrosis, while also improving glucose control. DA-1726 is a
novel oxyntomodulin (OXM) analogue that functions as a
glucagon-like peptide-1 receptor (GLP1R) and glucagon receptor
(GCGR) dual agonist. OXM is a naturally-occurring gut hormone that
activates GLP1R and GCGR, thereby decreasing food intake while
increasing energy expenditure, thus potentially resulting in
superior body weight loss compared to selective GLP1R agonists.
For more information, please visit www.neurobopharma.com.
Forward Looking Statements
Certain
statements in this press release may be considered forward-looking
statements within the meaning of the Private Securities Litigation
Reform Act of 1995. Words such as "believes", "expects",
"anticipates", "may", "will", "should", "seeks", "approximately",
"intends", "projects", "plans", "estimates" or the negative of
these words or other comparable terminology (as well as other words
or expressions referencing future events, conditions or
circumstances) are intended to identify forward-looking statements.
Forward-looking statements are predictions, projections and other
statements about future events that are based on current
expectations and assumptions and, as a result, are subject to risks
and uncertainties. Many factors could cause actual future events to
differ materially from the forward-looking statements in this press
release, including, without limitation, those risks associated with
NeuroBo's ability to execute on its commercial strategy; the
timeline for regulatory submissions; ability to obtain regulatory
approval through the development steps of NeuroBo's current and
future product candidates, the ability to realize the benefits of
the license agreement with Dong-A ST Co. Ltd., including the impact
on future financial and operating results of NeuroBo; the
cooperation of our contract manufacturers, clinical study partners
and others involved in the development of NeuroBo's current and
future product candidates; potential negative interactions between
our product candidates and any other products with which they are
combined for treatment; NeuroBo's ability to initiate and complete
clinical trials on a timely basis; our ability to recruit subjects
for its clinical trials; whether NeuroBo receives results from
NeuroBo's clinical trials that are consistent with the results of
pre-clinical and previous clinical trials; impact of costs related
to the license agreement, known and unknown, including costs of any
litigation or regulatory actions relating to the license agreement;
effects of changes in applicable laws or regulations; effects of
changes to NeuroBo's stock price on the terms of the license
agreement and any future fundraising; and other risks and
uncertainties described in our filings with the Securities and
Exchange Commission, including our most recent Annual Report on
Form 10-K. Forward-looking statements speak only as of the date
when made. NeuroBo does not assume any obligation to publicly
update or revise any forward-looking statements, whether as a
result of new information, future events or otherwise, except as
required by law.
Contact:
NeuroBo Pharmaceuticals,
Inc.
Marshall H.
Woodworth
Chief Financial Officer
+1-857-299-1033
marshall.woodworth@neurobopharma.com
Rx Communications Group
Michael Miller
+1-917-633-6086
mmiller@rxir.com
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/neurobo-pharmaceuticals-da-1241-in-combination-with-semaglutide-improves-liver-fibrosis-and-demonstrates-additive-hepatoprotective-effects-in-pre-clinical-mash-models-compared-to-either-treatment-alone-302152170.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/neurobo-pharmaceuticals-da-1241-in-combination-with-semaglutide-improves-liver-fibrosis-and-demonstrates-additive-hepatoprotective-effects-in-pre-clinical-mash-models-compared-to-either-treatment-alone-302152170.html
SOURCE NeuroBo Pharmaceuticals, Inc.