Hemogenyx Pharmaceuticals Says U.S. FDA Defers Pre-IND Meeting Until May
16 Fevereiro 2022 - 5:42AM
Dow Jones News
By Anthony O. Goriainoff
Hemogenyx Pharmaceuticals PLC said Wednesday that the U.S. Food
and Drug Administration has deferred a proposed
pre-investigational-new-drug, or pre-IND, meeting until May as it
is prioritizing work on Covid-19.
The London-listed biopharmaceutical group said the deferment
wasn't caused by any delay in the development of its lead product
candidate, Chimeric Antigen Receptor T-cells, known as
HEMO-CAR-T.
The company--which develops new therapies and treatments for
blood diseases--said that in the meantime HEMO-CAR-T's process will
be dealt with via written responses by the FDA to a submission the
company was in the process of refining. The company said a pre-IND
meeting will only be necessary if the written responses leave any
matters unresolved.
"The process toward our IND application remains on track and the
change in the FDA's process is not expected to affect the outcome
of the IND application or timetable for clinical trials," Chief
Executive Vladislav Sandler said.
Shares at 0801 GMT were down 0.05 pence, or 3%, at 1.63
pence.
Write to Anthony O. Goriainoff at
anthony.orunagoriainoff@dowjones.com
(END) Dow Jones Newswires
February 16, 2022 03:27 ET (08:27 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
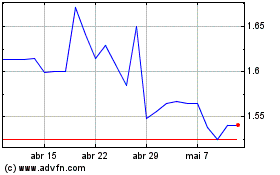
Hemogenyx Pharmaceuticals (LSE:HEMO)
Gráfico Histórico do Ativo
De Abr 2024 até Mai 2024
Hemogenyx Pharmaceuticals (LSE:HEMO)
Gráfico Histórico do Ativo
De Mai 2023 até Mai 2024