Regenxbio's RGX-121 Lands Regenerative Medicine Advanced Therapy Designation
23 Maio 2023 - 8:55AM
Dow Jones News
By Chris Wack
Regenxbio said Tuesday that the U.S. Food and Drug
Administration has granted a regenerative medicine advanced therapy
designation for RGX-121, an investigational one-time AAV
therapeutic for the treatment of mucopolysaccharidosis Type II,
also known as MPS II or Hunter syndrome.
RMAT designation is designed to expedite the drug development
and review processes for promising new treatments, including gene
therapies. The designation recognizes that the preliminary clinical
evidence from RGX-121 demonstrates its potential to address unmet
medical needs for MPS II.
Regenxbio said RGX-121 is currently being studied in a trial
that is enrolling MPS II patients. The company continues to support
plans to file a biologics license application for RGX-121 in 2024
using the accelerated approval pathway.
A drug is eligible for RMAT designation if it is intended to
treat, modify, reverse or cure a serious or life-threatening
disease or condition, and if preliminary clinical evidence
indicates the drug or therapy has the potential to address unmet
medical needs for such disease or condition.
A Phase I/II trial of RGX-121 for the treatment of MPS II in
pediatric patients over the age of five years old is ongoing.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
May 23, 2023 07:40 ET (11:40 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
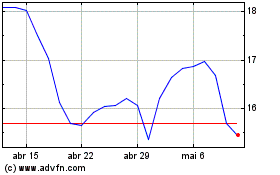
REGENXBIO (NASDAQ:RGNX)
Gráfico Histórico do Ativo
De Abr 2024 até Mai 2024
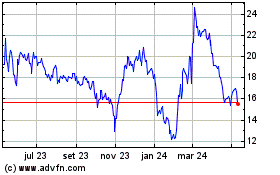
REGENXBIO (NASDAQ:RGNX)
Gráfico Histórico do Ativo
De Mai 2023 até Mai 2024