Novartis's Sandoz Gets FDA Approval for Multiple Sclerosis Biosimilar
25 Agosto 2023 - 3:02AM
Dow Jones News
By Adria Calatayud
Novartis's Sandoz generic-drugs unit has received approval from
the U.S. Food and Drug Administration for biosimilar medicine
Tyruko to treat relapsing forms of multiple sclerosis.
Sandoz said Friday that the drug--which was developed by
Polpharma Biologics and for which the Swiss company entered into a
global commercialization agreement in 2019--is approved to treat
all indications covered by reference medicine Tysabri for relapsing
forms of multiple sclerosis. Biosimilars are near-replicas of
already approved biologic drugs.
This makes Tyruko the first FDA-approved biosimilar for
relapsing forms of multiple sclerosis, Sandoz said. The approval
was based on robust Phase 1 and 3 clinical studies, the company
said.
Write to Adria Calatayud at adria.calatayud@dowjones.com
(END) Dow Jones Newswires
August 25, 2023 01:47 ET (05:47 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
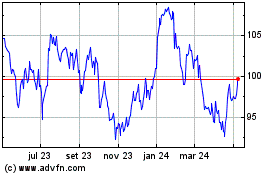
Novartis (NYSE:NVS)
Gráfico Histórico do Ativo
De Jun 2024 até Jul 2024
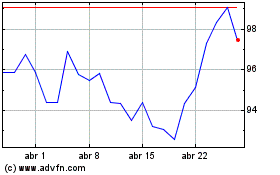
Novartis (NYSE:NVS)
Gráfico Histórico do Ativo
De Jul 2023 até Jul 2024