Ligand Pharmaceuticals Gets FDA OK for Viral Skin Infection Treatment
05 Janeiro 2024 - 7:40PM
Dow Jones News
By Ben Glickman
Ligand Pharmaceuticals said its treatment for molluscum, a viral
skin infection, had received approval from U.S. regulators.
The company said Friday that the U.S. Food and Drug
Administration had approved Zelsuvmi, a topical prescription
medication, to treat moluscum in adults and children one year old
or older.
Ligand expects the treatment to be available in the U.S. in the
second half of 2024.
The company said the FDA had approved Zelsuvmi as the first
novel drug to treat mollescum infections.
Molluscum is a contagious skin infection which can cause
lesions. It primarily affects children.
Write to Ben Glickman at ben.glickman@wsj.com
(END) Dow Jones Newswires
January 05, 2024 17:25 ET (22:25 GMT)
Copyright (c) 2024 Dow Jones & Company, Inc.
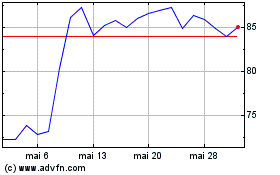
Ligand Pharmaceuticals (NASDAQ:LGND)
Gráfico Histórico do Ativo
De Abr 2024 até Mai 2024
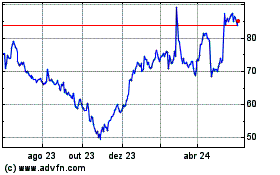
Ligand Pharmaceuticals (NASDAQ:LGND)
Gráfico Histórico do Ativo
De Mai 2023 até Mai 2024