Filed by A SPAC I Mini Acquisition Corp.
pursuant to Rule 425 under the Securities Act of 1933
and deemed filed pursuant to Rule 14a-12
under the Securities Exchange Act of 1934
Subject Company: A SPAC I Acquisition Corp.
Commission File No.: 001-41285

Company Investor Presentation July 2023

2 Disclaimer This confidential presentation (the “Presentation”) is being delivered to a limited number of parties for discussion purposes on ly. Any reproduction of distribution of this Presentation, in whole or in part, or the disclosure of its contents, without the prior consent of A SPAC I Acquisition Corp (“ASCA”) or NewGenIvf Limited (the “Company”) is prohibited and may also be restricted by law and persons into whose possession this Presentation c om es should inform themselves about and observe any such restrictions. By accepting this Presentation, each recipient agrees: ( i ) to maintain the confidentiality of all information that is contained in this Presentation and not already in the public domain; and (ii) to use this Presentation for information purposes only and not as the basis for any voting or investment dec isi on with respect to ASCA or the Company. This Presentation does not purport to contain all of the information that may be required to evaluate a possible voting or in ves tment decision with respect to ASCA or the Company. The recipient agrees and acknowledges that ( i ) it is aware that the United States securities laws prohibit any person who has material, non - public information concerning a company f rom purchasing or selling securities of such company or from communicating such information to any other person under circumstances in which it is reasonably foreseeable that such person is likely to purchase or sell such se cur ities, (ii) it is familiar with the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder (collectively, the "Exchange Act"), and that the recipient will not use, and will cause an y t hird party not to use, this Presentation or any information contained herein in contravention of the Exchange Act, including, without limitation, Rule 10b - 5 thereunder, and (iii) this Presentation is not intended to form the basi s of any voting or investment decision by the recipient and does not constitute investment, tax or legal advice. No representation or warranty, express or implied, is or will be given by ASCA or the Company or any of their respect ive affiliates, directors, officers, employees or advisers or any other person as to the accuracy, completeness or reliability of the information in this Presentation or any other written, oral or other communications transm itt ed or otherwise made available to any party in the course of its evaluation of the proposed business combination between ASCA and the Company (the “Business Combination”), and no responsibility or liability whatsoever is accep ted for the accuracy or sufficiency thereof or for any errors, omissions or misstatements, negligent or otherwise, relating thereto. The recipient also acknowledges and agrees that the information contained in this Presentation i s p reliminary in nature and is subject to change, and any such changes may be material. ASCA and the Company disclaim any duty to update the information contained in this Presentation. Forward - Looking Statements This Presentation and oral statements made in any meeting include “forward - looking statements” within the meaning of the “safe harbor ” provisions of the Private Securities Litigation Reform Act of 1996. The Company’s actual results may differ from expectations, estimates and projections and consequently, you should not rely on these forward - looking s tatements as predictions of future events. Words such as “objective", “expect”, “estimate”, “project”, “budget”, “forecast”, “anticipate”, “intend”, “plan”, “may”, “will ”, ”might”, “can”, “could”, ”should”, “believe”, “predict” , “ potential”, “continue”, and similar expressions suggest future outcomes and are intended to identify such forward - looking statements. These forward - looking statements include, without limitation, the Company’s and ASCA’s expectations with res pect to anticipated financial impacts of the Business Combination, opinions and estimates of management at the date the statements are made, the satisfaction of closing conditions to the Business Combination, the timin g o f the completion of the Business Combination and future performance of the parties thereto. These forward - looking statements are subject to a variety of risks and uncertainties and other factors that could cause the actual eve nts or results to differ materially from the expected results and the forward - looking statements. Although the Company believes that the expectations reflected in the forward - looking statements are reasonable, there can be no assurance that such expectations will prove to be correct. The Company cannot guarantee future results, level of activity, performance or achievements and there is no representation that the actual results achieved will be the same, in wh ole or in part, as those set out in the forward - looking statements. By their nature, forward - looking statements involve numerous assumptions, known and unknown risks and uncertainties, both general and specific, that contribut e t o the possibility that the predictions, forecasts and other forward - looking information will not occur, which may cause the Company’s actual performance and financial results in future periods to differ materially from any estima tes of future performance, illustrations of performance results or results expressed or implied by such forward - looking statements. Factors that may cause such differences include, but are not limited to: (1) the inability of ASCA a nd/or the Company to complete the Business Combination due to the failure to obtain approval of the shareholders of ASCA, to have sufficient cash available to complete the Business Combination or to satisfy other conditions t o c losing; (2) matters discovered by ASCA or the Company as they complete their respective due diligence investigations of each other; (3) the outcome of any legal proceedings that may be instituted against ASCA, the Com pan y or others following announcement of the Business Combination and any definitive agreement with respect thereto; (4) the risk that the announcement or consummation of the Business Combination disrupts current plans and op era tions; (5) the inability to recognize the anticipated benefits of the Business Combination; (6) costs related to the Business Combination; (7) changes in the applicable laws or regulations, including but not limited to IVF and sur rogacy laws and regulations; (8) other risks and uncertainties indicated from time to time in ASCA periodic reports filed with the SEC and other filings with the SEC in connection with the Business Combination; (9) the occur ren ce of any event, change or other circumstances that could give rise to the termination of any definitive agreements with respect to the Business Combination; (10) changes to the proposed structure of the Business Combination that may be required or appropriate as a result of applicable laws or regulations or as a condition to obtaining regulatory approval of the Business Combination; and (11) the ability to meet stock exchange listing standards foll owi ng the consummation of the Business Combination. ASCA and the Company caution that the foregoing list of factors is not exclusive and readers shall not place undue reliance upon any forward - looking statements. Except as requi red by law, neither ASCA nor the Company undertakes or accepts any obligation to release publicly any updates or revisions to any forward - looking statements to reflect any changes in its expectations or any change in events, condi tions or circumstances on which any such statement is based. Industry and Market Data In this Presentation, the Company and ASCA rely on and refer to information and statistics regarding market participants in t he sectors in which the Company competes and other industry data. The Company obtained this information and statistics from third - party sources, including reports by market research firms and filings of other companies i n the same industry.

3 Disclaimer (Continued) Trademarks This Presentation may contain trademarks, service marks, trade names and copyrights of other companies, which are the propert y o f their respective owners. Solely for convenience, some of the trademarks, service marks, trade names and copyrights referred to in this Presentation may be listed without the TM, SM or ® Œ symbols, but ASCA and the Company will assert, to the fullest extent under applicable laws, the rights of the applicable owne rs , if any, to these trademarks, service marks, trade names and copyrights. No Offer or Solicitation This Presentation shall not constitute a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the Business Combination. This Presentation shall also not constitute any offer to sell, a solicitation of an offer to buy or recommendation to purchase any securities, nor shall there be any sale of securities in an y s tates or jurisdictions in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a p rospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended, or an exemption therefrom. You should not construe the contents of this Presentation as legal, tax, accounting or investment advice or a recommendation. You should consult your own counsel and tax and financial advisors as to legal and related matters concerning the matters described herein, and, by accepting this Presentation, you confirm that you are no t r elying upon the information contained herein to make any decision. Financial Information Unless otherwise specified, the financial information and data contained in this Presentation is unaudited, is based on draft st atutory accounts, does not conform to Regulation S - X, and is subject to Public Company Accounting Oversight Board audit, with respect to yearly data, and subject to audit review, with respect to semi - annual data. Accordingly, such information and data may not be included in, may be adjusted in or may be presented differently in the registration statement (the “Registration Statement”), to be filed with the SEC in connection with the Business Combinati on and the proxy statement/prospectus contained therein. You should review the Company's audited financial statement, which will be include in the Registration Statement relating to the proposed Business Combination. In ad dit ion, all of the Company’s historical financial information included herein is preliminary and subject to change. Important Information and Where to Find It This document does not contain all the information that should be considered concerning the proposed Business Combination. It do es not constitute an offer to sell or exchange, or the solicitation of an offer to buy or exchange, any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, sale or exchange would be unla wfu l prior to registration or qualification under the securities laws of any such jurisdiction. It is not intended to form the basis of any investment decision or any other decision in respect of the proposed Business Combination. In connectio n w ith the proposed Business Combination, the successor public entity intends to file with the SEC a registration statement on Form F - 4, which will include a preliminary proxy statement/prospectus with respect to the Business Com bination. When available, the definitive proxy statement/prospectus and other relevant materials for the proposed Business Combination will be mailed to ASCA shareholders as of a record date to be established for purposes of v oti ng on the proposed Business Combination. ASCA shareholders and other interested persons are advised to read, when available, the preliminary proxy statement/prospectus and any amendments thereto, and the definitive pr oxy statement/prospectus and other documents filed and to be filed in connection with the proposed Business Combination, as these materials contain and will contain important information about the Company, ASCA and the propo sed Business Combination. Shareholders will also be able to obtain copies of the preliminary proxy statement/prospectus and the definitive proxy statement/prospectus and other documents filed with the SEC, once they are avai lab le, without charge, at the SEC’s website at www.sec.gov or by directing a request to: A SPAC I Acquisition Corporation, Level 39, Marina Bay Financial Centre, Tower 2, 10 Marina Boulevard, Singapore, 018983. INVESTMENT IN ANY SECURITIES DESCRIBED HEREIN HAS NOT BEEN APPROVED OR DISAPPROVED BY THE SEC OR ANY OTHER REGULATORY AUTHORI TY NOR HAS ANY AUTHORITY PASSED UPON OR ENDORSED THE MERITS OF THE OFFERING OR THE ACCURACY OR ADEQUACY OF THE INFORMATION CONTAINED HEREIN. ANY REPRESENTATION TO TH E C ONTRARY IS A CRIMINAL OFFENSE. Participants in the Solicitation ASCA, the Company and their respective directors and executive officers, other members of management and employees may be con sid ered participants in the solicitation of proxies with respect to the potential Business Combination described in this Presentation under the rules of the SEC. Information about the directors and executive officers of ASCA is set forth in ASCA’s Annual Report on Form 10 - K for the year ended December 31, 2022, which was filed with the SEC on March 3, 2023. Information regarding other persons who may, under the rules of the SEC, be de eme d participants in the solicitation of the shareholders in connection with the potential transaction and a description of their interests will be set forth in the Registration Statement when it is filed with the SEC. These doc ume nts can be obtained free of charge from the sources indicated above.

4 01 Executive Summary 02 Industry Outlook 03 Investment Highlights and Company Strategy 04 Business Operations 05 Company Financials 06 Appendix & Risk Factors

1 Executive Summary 1

6 Transaction Overview Background Pro Forma Ownership & Valuation Alignment of Equity Interest Governance NewGenIvf Limited (“ NewGenI vf ”) entered into a merger agreement with A SPAC I Acquisition Corp. (“ASCA”) on February 15, 2023 NewGenIvf management and shareholders to own 45% at close of the merger, assuming no redemptions and no exercise of warrants NewGenIvf 2022 year - end fair market value of US $54,200,000 Certain shareholders of NewGenIvf will enter into a 1 - year lock - up ─ I f 20 - Day VWAP exceeds $15.0 over any 30 - day trading period, 20% of lock - up shares can be subject to early release The Board of PubCo will consist of (7) directors ─ NewGen Ivf will elect (3) Executive Directors prior to the closing ─ NewGen Ivf will elect another ( 4) Independent Directors

7 Company Overview Stable Profitability During COVID Despite Challenging Environment Legally - Qualified & Licensed Provision of IVF & Surrogacy Services 3 Clinics at Favourable Locations with Multi - lingual & Multi - cultural Staff Fast Growing Market Size With Significant Unmet Demand For ARS Comprehensive ARS Solution Provider With IVF & Surrogacy Services Exclusively ─ Licensed MicroSort Technology 1 For Gender Selection and Screening Note : (1) In Thailand and Cambodia

1 Industry Overview 2

9 2.1 ARS: Rising Demand With Significant Growth Opportunity Note : (1) In 2022 Source: F rom research conducted by China Insights Consultancy (“CIC”) Shifting Social and Cultural View towards ARS Continued de - stigmatization of infertility drive stronger demand for fertility treatment services Improving living standards and awareness about birth defects and prevention Policy Relaxation towards Marriage & Birth Growing Legal Recognition of Same - Sex Marriages China: Implementation of Three - Child Policy in May 2021 Rising Infertility Rate in Asia - Pacific 1 Increasing Awareness Improved Understanding Increased Affordability of ARS More Women of Reproductive Age in Asia - Pacific Thailand 15.4% India 13.8% China 17.8% R ising Demand For Fertility Services 816.4 833.2 2014 2022 (in Million)

10 2.2 ARS: Expanding Market Size And Unfulfilled Market Demand 213+ million Infertile couples in Asia - Pacific in 2022 US$37.4 bn Potential Asia Fertility Market in 2030 Rising ARS Users in Recent Years 98.9% Untreated Infertile Couples 1 China (000’ cases) Japan (000’ cases) 136.8 184.9 2017 2022 98.0 128.5 2017 2022 1.47 m ARS cycle performed 1 , account for only 1.1% of infertility base Note : (1) In year 2022 Source: From research conducted by CIC

1 Investment Highlights and Company Strategy 3

12 3.1 Investment Highlights Solution Provider Offering Comprehensive Fertility Treatment Services • IVF services in Thailand and Cambodia • Greater affordability 1 Attractive Market with Significant Demand and Fast Growth Exclusively Licensed Use of “ MicroSort 2 ” & Access to Latest Fertility Technologies • High entry barrier with heavy regulation and stringent regulatory requirements to obtain licenses • In Thailand & Cambodia: Exclusive license to use MicroSort technology • Adoption of latest technology: PGS, NGS & PGD Well Established Brand with Reliable Reputation Experienced Management Team • CEO, Mr. Alfred Siu: 13+ years of experience • Dr. Wiphawee Luangtangvarodom : 10+ years of experience as an obstetrician and gynecologist • Ms. Anussara Phinyong and Ms. Araya Boonchaisitthipong : each with 8+ years of experience in the embryologist field Note: (1) Average cost per IVF cycle in the US is US$12,000, while it is US$7,000 for NewGen Ivf (ex. Medication) (2) In Thailand and Cambodia • Legally - qualified & licensed provision of surrogacy services in Kyrgyzstan • Multi - lingual and multi - cultural staff • Growing approx. at 15% YoY in Asia - Pacific in 2022 • Substantial untreated & underserved patients • Supply constraints due to heavy regulation & stringent requirements • Relaxed birth regulation • Increased awareness & acceptance of IVF & surrogacy • Founders entered the fertility market in 2010 • Relatively scalable operating model with standardized workflows & operating procedures • Favourable geographic locations of multiple clinics

13 3.2 Company Strategy Offer Broad Fertility Services for Fertility Tourists across Asia - Pacific Integrate fertility services with travel - related offerings to enhance customer’s experience Aim to capture a share of the growing market for fertility tourism in Asia - Pacific Continue to Invest in Laboratories and Facilities Updates Scale up existing labs and facilities to support research and development, e.g. improved ARS success rates and lower costs Develop clinically customized interior design concepts Increase Brand Awareness and Market Share Collaborate with hospitality providers and utilize social media promotions to attract clients Establish a business development team and introduce innovative treatment services to strengthen reputation in growing ARS market in Asia - Pacific Expand Service Reach Through Acquisitions and Partnerships Focus on acquiring ARS providers in Asia - Pacific with recognized brands and licenses Partner with corporate clients to offer corporate benefit programs and partnerships to offer fertility services to companies with female staff

1 Business Operations 4

15 4.1 Corporate History 2010 IVF agency: Sending patients to Thailand 2014 Register IVF Center First Firtility PGS Center in Bangkok Thailand 2015 Register First Fertility Phnom Penh Limited in Phnom Penh Cambodia 2019 Register First Fertility Bishkek in Kyrgyzstan First Fertility PGS Center Bangkok Thailand accredited by Joint Commission International (JCI) Established NewGenIvf Cayman Islands Holding Company PRESENT New Chapter Awards & Recognitions NewGenIvf Clinics & Labs Prime location central Bangkok

16 Surrogacy Services Maternity care services, including body check, provision of vitamins, supplements & medicines Birth certificate application Infant’s passport & visa services Full - Cycle Services (IVF) Consultation, tests & treatment plan design Synthetic hormones injection & regular monitoring Ovarian stimulation & trigger injection Trans Vaginal Follicle Aspiration surgery Consultation, Body Check & Basic Services (IVF) Consultation, health examination & evaluation Acupuncture & traditional Chinese Medicine 4.2 Provision of A Broad Spectrum of ARS Products & Services Intracytoplasmic sperm injection Embryos fertilized, biopsied and tested Embryos transfer Egg - Freezing Services (IVF) Consultation, tests & treatment plan design Synthetic hormones injection & regular monitoring Ovarian stimulation & trigger injection Trans Vaginal Follicle Aspiration surgery Making Parenthood Dream Come True Through a Supported Fertility Journey
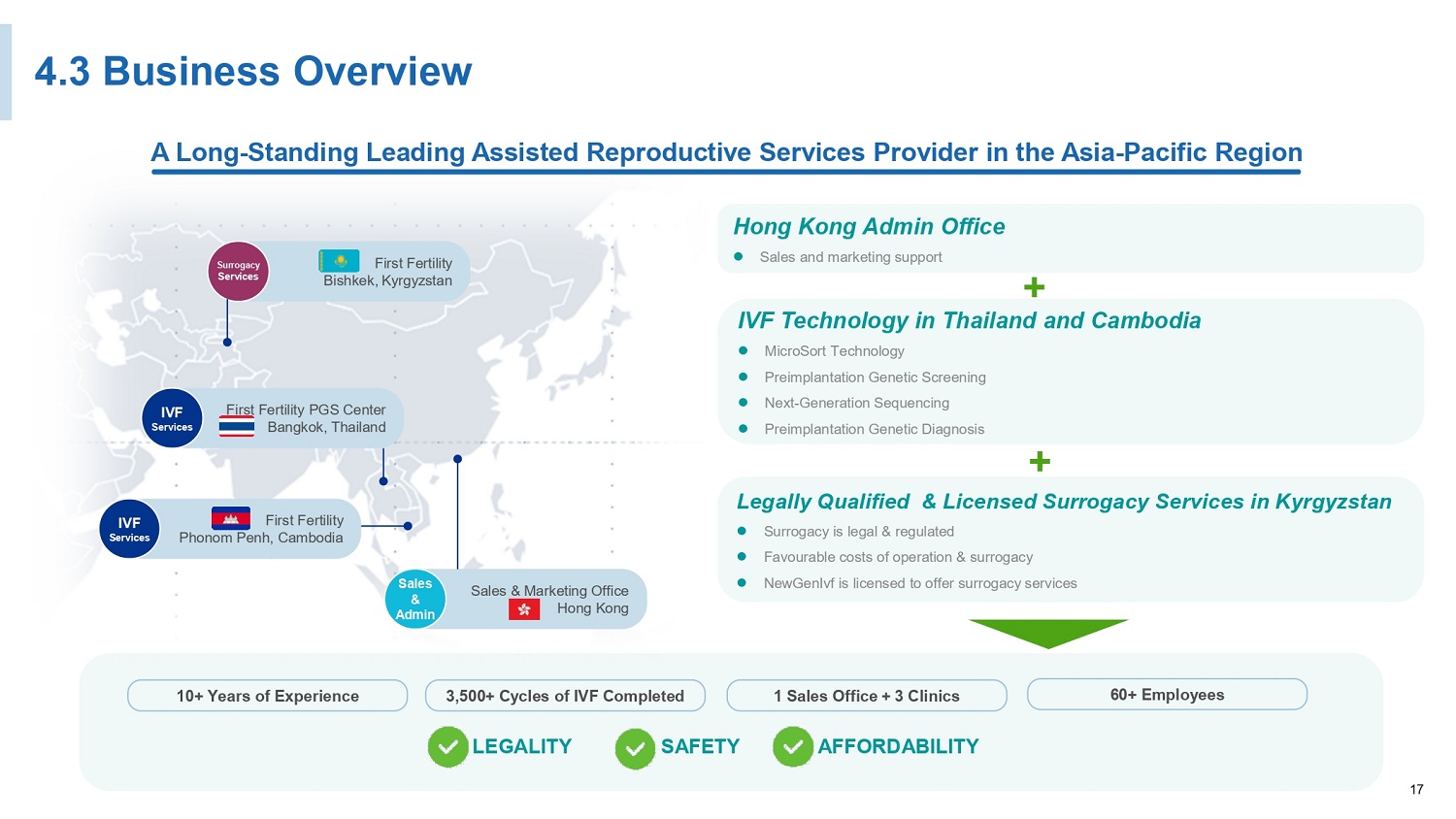
17 4.3 Business Overview 10+ Years of Experience 3,500+ Cycles of IVF Completed 1 Sales Office + 3 Clinics 60+ Employees First Fertility PGS Center Bangkok, Thailand IVF Services First Fertility Phonom Penh, Cambodia IVF Services Sales & Marketing Office Hong Kong Sales & Admin First Fertility Bishkek, Kyrgyzstan Surrogacy Services A Long - Standing Leading Assisted Reproductive Services Provider in the Asia - Pacific Region LEGALITY SAFETY AFFORDABILITY Legally Qualified & Licensed Surrogacy Services in Kyrgyzstan Surrogacy is legal & regulated Favourable costs of operation & surrogacy NewGenIvf is licensed to offer surrogacy services IVF Technology in Thailand and Cambodia MicroSort Technology Preimplantation Genetic Screening Next - Generation Sequencing Preimplantation Genetic Diagnosis + Hong Kong Admin Office Sales and marketing support +

18 4.4 Business Operations Continuing Effective Marketing • Referrals • Social media promotions and marketing initiatives • Strategic investments & partnerships Experienced Physicians & Medical Professionals • Medical professionals • Nursing professionals • Medical technology professionals Licenses & Approvals Accredited Medical Facilities Across Various Location • Thailand ─ 6,582sqft • Cambodia ─ 7,535sqft • Kyrgyzstan ─ 1,615sqft Advanced Technology for Tailored ARS services • MicroSort Technology 1 • Preimplantation Genetic Screening (“PGS”) • Next - Generation Sequencing (“NGS”) • Preimplantation Genetic Diagnosis (“PGD”) The Ministry of Health of the Kyrgyz Republic The Ministry of Health of Thailand The Minist ry of Health, Kingdom of Cambodia Note : (1) In Thailand and Cambodia

19 MicroSort Technology Microsort Technology Traditional Method Microsort Technology 4.5 Exclusive License to Use MicroSort in Thailand & Cambodia VS Process of MICROSORT A p re - conception gender selection technology , which means: Gender selection is conducted post - conception Conceive Filter Select 1 2 3 Filter Conceive Select 1 2 3 Eggs are more likely to be fertilized according to the preferences of the parents sort the sperm cells by flow cytometer Gender selection happens after conception, meaning some fertilized eggs will go unused

20 4.6 Surrogacy Procedure and Timeline STEP 1 STEP 2 STEP 3 STEP 4 STEP 5 STEP 6 Frozen Embryo Transfer • Arrange for the surrogate mother (who has already been tested and qualified) for embryo transfer; all surrogate mothers will be under the care of First Fertility Bishkek . Pregnancy Test • If the result of the pregnancy test 10 days after embryo transfer is positive, the couple need to fly to Kyrgyzstan to prepare documents for surrogacy, which will take 2 - 3 days. Documentation • Once the documents are completed, the surrogate will start having all required check - ups; an update report will be provided by First Fertility Bishkek. Infant Delivery • When the infant is nearing delivery, the couple (husband or wife) need to be in Kyrgyzstan to await the delivery. Documents for the Infant • Local lawyer will assist with the birth certificate and facilitate applying for the infant’s passport and visa (approx. 1 month) Bringing Infant Home • When all the documents are completed, the infant can be brought home

21 4.7 Management Team Photo to come Mr. Siu Wing Fung, Alfred Founder, Chairman of the Board of Directors Ms. Fong Hei Yue, Tina Director, Co - Founder, Chief Marketing Officer • Has served as the Chairman of the Board and CEO of NewGenIvf since 2019 • Served as the director of First Fertility PGS Center Co., Ltd since 2014 • Received his master’s and bachelor’s degree in Science from Stanford University • Has served as the Director and Chief Marketing Officer of NewGenIvf since 2019 • Served as the director of First Fertility PGS Center Co., Ltd since 2014 • Received her bachelor’s degree in marketing from Indiana University

1 Company Financials 5

23 5.1 Robust Growth in Revenue With Stable Profit Margin Robust Revenue Growth (in US$’000) Revenue Breakdown by Geographic Regions (in US$’000) 693.9 Thailand 313.7 Cambodia 3,110.5 Kyrgyzstan 2021 505.6 Thailand 377.6 Cambodia 5,061.0 Kyrgyzstan 2022 Gross Profit and Gross Profit Margin (in US$’000) 1,024.8 1,537.8 24.9% 25.9% 22.0% 0 500 1000 1500 2000 2021 2022 Gross Profit Gross Profit Margin 0 1000 2000 3000 4000 5000 6000 7000 2021 2022 Revenues 4,118.1 5,944.2 Significant Growth in Surrogacy Services and Ancillary Caring Services 918.4 3,125.0 0.0 500.0 1,000.0 1,500.0 2,000.0 2,500.0 3,000.0 3,500.0 2021 2022 Surrogacy & Ancillary Caring Services (in US$’000)

24 5.2 Effective Expense Control and Rising Profitability Significant Rise in Net Income Attributable to Shareholders and EPS (in US$’000 for Net Income, in US$ for EPS) 199.5 698.7 4.8% 11.8% 0.0% 2.0% 4.0% 6.0% 8.0% 10.0% 12.0% 14.0% 16.0% 18.0% 20.0% 0 100 200 300 400 500 600 700 800 2021 2022 Net Income Attributable to Shareholders Net Income to Revenue Ratio EPS 0.36 EPS 1.21 Operating Expense and Operating Expense to Revenue Ratio (in US$’000) 626.0 899.1 15.2% 15.1% 10.0% 0 100 200 300 400 500 600 700 800 900 1000 2021 2022 Operating Expense Operating Expense to Revenue Ratio

Appendix

26 Appendix 1: IVF Procedure

Risk Factors

28 Risk Factors References in the summary below to “ASCA” refer to A SPAC I Acquisition Corp., which will reincorporate to the British Virgin Is lands by merging with and into A SPAC I Mini Acquisition Corp. (“ PubCo ”), with PubCo remaining as the surviving publicly traded entity. The combined company after the Business Combination is referred to as the “Combined Company.” Referen ces to “ NewGenIvf ” or the "Company" generally refer to NewGenIvf Limited in the present tense or to the Combined Company from and after the Business Combination. The following summarizes certain principal factors that make an investment i n t he Combined Company speculative or risky, all of which are more fully described in the “Risk Factors” section in the registration statement to be filed with the SEC in connection with the Business Combination and the proxy statement/prosp ect us contained therein. This summary should be read in conjunction with the “Risk Factors” section, together with the information in the Company’s consolidated financial statements and related notes, and should not be relied upon as an exhaust ive summary of the material risks facing ASCA’s NewGenIVF’s and/or the Combined Company’s business. Risks Related to NewGenIvf’s Business and Industry • The fertility market in which NewGenIvf participates is competitive, and if NewGenIvf does not continue to compete effectively, its results of operations could be harmed. • NewGenIvf has a limited operating history with its current platform of solutions, which makes it difficult to predict its future prospe ct s, financial performance and results of operations. • NewGenIvf’s marketing efforts depend significantly on its ability to receive positive references from its existing clients. • If NewGenIvf is unable to attract new clients, its business, financial condition and results of operations would be adversely affected. • NewGenIvf’s business depends on its ability to maintain its existing client demographics. Any failure to do so would harm its business, f in ancial condition and results of operations. • A significant reduction in the utilization of NewGenIvf’s solutions could have an adverse effect on its business, financial condition and results of operations. • If NewGenIvf fails to offer high - quality support, its reputation could suffer. • Failure to effectively develop and expand its marketing and sales capabilities could harm its ability to increase its client bas e and achieve broader market acceptance of solutions NewGenIvf provides. • NewGenIvf may experience net losses and may not sustain profitability in the future. • NewGenIvf’s future revenue may not grow at the rates it historically has, or at all. • NewGenIvf’s quarterly and annual results may fluctuate significantly and may not fully reflect the underlying performance of NewGenIvf’s business. • If the estimates and assumptions NewGenIvf uses to determine the size of the target markets for its services are inaccurate, its future growth rate may be impacted and it s business would be harmed. • NewGenIvf may not be able to successfully manage its growth, and if NewGenIvf is not able to grow efficiently, its business, financial condition and results of operations could be harmed. • If NewGenIvf’s new solutions and services are not adopted by its clients, or if it fails to innovate and develop new offerings that are adop te d by its clients, its revenue and results of operations may be adversely affected. • If NewGenIvf fails to adapt and respond effectively to the changing medical landscape, changing regulations, changing client needs, requir em ents or preferences, its offerings may become less competitive. • If NewGenIvf fails to maintain and enhance its brand, its ability to expand its client base will be impaired and its business, financial c on dition and results of operations may suffer. • If NewGenIvf fails to retain and motivate members of its management team or other key employees, or fails to attract additional qualified pe rsonnel to support its operations, its business and future growth prospects could be harmed. • To successfully market and sell its services and products in Asia - Pacific markets, NewGenIvf must address many international business risks with which NewGenIvf has limited experience. • Ethical, legal and social concerns related to the use of assisted reproductive technology could reduce demand for the fertili ty services provided by the medical facilities in NewGenIvf’s network, and thus may adversely affect the business, financial conditions and results of operations of the medical facilities in its network. • NewGenIvf is reliant on revenue from international clients. • Fluctuations in exchange rates could have a material and adverse effect on NewGenIvf’s results of operations and the value of your investment. • Governmental control of currency conversion may limit NewGenIvf’s ability to utilize NewGenIvf’s net revenue effectively and affect the value of your investment. • Substantially all of NewGenIvf’s assets and operations are located in Thailand, Cambodia and Kyrgyzstan and they are subject to economic, legal and regulatory u ncertainties in such countries. • Failure to comply with the terms of future financing arrangements could result in default, which could have an adverse effect on NewGenIvf’s cash flow and liquidity. • NewGenIvf requires a significant amount of capital to fund its operations and growth. If NewGenIvf cannot obtain sufficient capital on acceptable terms, its business, financial condition, and prospects may be materially and ad versely affected. • The defects in certain leased property interests and failure to register certain lease agreements may materially and adversel y a ffect NewGenIvf’s business, financial condition, results of operations, and prospects. • NewGenIvf has limited insurance coverage for its operations. • NewGenIvf may not be successful in adapting to technological developments, which may affect its business and results of operations. • If its computer systems, or those of its providers, specialty pharmacies or other downstream vendors lag, fail or suffer sec uri ty breaches, NewGenIvf may incur a material disruption of its services, which could materially impact its business and the results of operations.

29 Risk Factors (Continued) Risks Related to NewGenIvf’s Relationships with Third Parties • NewGenIvf’s business depends on its ability to maintain its network of high - quality fertility specialists and other healthcare providers. I f NewGenIvf is unable to do so, its future growth would be limited and its business, financial condition and results of operations would be harmed. • The medical facilities and professionals in NewGenIvf’s network could become the subject of litigation, allegations and other claims, and NewGenIvf may not be adequately insured against these liabilities. • The assisted reproductive medical facilities in NewGenIvf’s network have limited control over the quality of the pharmaceuticals, medical equipment, medical consumables and other suppli es used in its operations, and cannot guarantee that the products in use are not defective or counterfeit. • If NewGenIvf loses its relationship with one or more key pharmaceutical manufacturers, its business and results of operations could be adv er sely affected. • NewGenIvf has engaged in transactions with related parties, and such transactions present potential conflicts of interest that could ha ve an adverse effect on its business and results of operations. • NewGenIvf may be subject to claims and allegations relating to intellectual property and other causes. • Certain data and information in this proxy statement/prospectus relied on NewGenIvf were obtained from third - party data and polls. These metrics were not independently verified by NewGenIvf and may not be accurate. Risks Related to Government Regulation • NewGenIvf operates in a highly regulated industry and must comply with a significant number of complex and evolving requirements. Any l ac k of requisite approvals, licenses, or permits applicable to NewGenIvf’s business may have a material and adverse impact on NewGenIvf’s business, financial condition, and results of operations. • Legal or regulatory restriction, government regulation, industry standards and other requirements create risks and challenges wi th respect to NewGenIvf’s compliance efforts and its business strategies and could adversely impact NewGenIvf’s business and limited the growth of NewGenIvf’s operations. • Any litigation against it could be costly and time - consuming to defend and could harm NewGenIvf’s business, financial condition and results of operations. • Acquisitions, strategic investments, partnerships, or alliances could be difficult to identify, pose integration challenges, div ert the attention of management, disrupt NewGenIvf’s business, dilute stockholder value, and adversely affect its business, financial condition and results of operations. • Changes in NewGenIvf’s effective tax rate or tax liability may have an adverse effect on its results of operations. • NewGenIvf’s reported financial results may be adversely affected by changes in accounting principles generally accepted in relevant juris di ctions. • If NewGenIvf’s estimates or judgments relating to its critical accounting policies prove to be incorrect, its results of operations could b e adversely affected. • NewGenIvf is subject to anti - corruption, anti - bribery, anti - money laundering, and similar laws, and non - compliance with such laws can sub ject it to criminal or civil liability and harm its business, financial condition and results of operations. Risks Related to ASCA’s Business and the Business Combination • If ASCA is deemed to be an investment company for purposes of the Investment Company Act, ASCA would be required to institute bu rdensome compliance requirements and its activities would be severely restricted and, as a result, ASCA would likely abandon its efforts to consummate an initial business combination and liquidate and dissolve. • To mitigate the risk that ASCA might be deemed to be an investment company, ASCA may, at any time, instruct the trustee to l iqu idate the securities held in the Trust Account and instead to hold the funds in the Trust Account in cash, resulting in ASCA receiving minimal interest and reducing the dollar amount the public shareholders would receive upon any redemption or liquid ati on of ASCA. • ASCA’s independent registered public accounting firm’s report contains an explanatory paragraph that expresses substantial do ubt about ASCA’s ability to continue as a “going concern;” • You must tender your ASCA Class A ordinary shares in order to validly seek redemption at the Meeting. • If third parties bring claims against ASCA, the proceeds held in trust could be reduced and the per - share liquidation price rece ived by ASCA’s shareholders may be less than $10.10. • Any distributions received by ASCA shareholders could be viewed as an unlawful payment if it was proved that immediately foll owi ng the date on which the distribution was made, the value of ASCA’s assets did not exceed its liabilities or ASCA was unable to pay its debts as and when they fell due. • ASCA will be forced to liquidate the Trust Account if it cannot consummate a business combination by July 17, 2023 (unless ex ten ded monthly up to October 17, 2023 as allowed under its Existing Charter). In the event of a liquidation, ASCA’s public shareholders will receive approximately $[•] per share. • ASCA will incur significant transaction costs in connection with transactions contemplated by the Merger Agreement. Risks PubCo’s Securities Following the Business Combination and PubCo Operating as a Public Company • PubCo may not qualify as, or continue to satisfy the requirement for, a foreign private issuer, which may require PubCo to fully comply with more stringent reporting requirements of the Exchange Act for domestic issuers. • PubCo is incorporated under British Virgin Islands law, and investors may face difficulties protecting its rights in the U.S. and u nd er U.S. Law. • There are, and continue to be, uncertainties involving the PubCo’s status under U.S. tax law which may adversely affect PubCo’s financial operation.

Q&A

THANK YOU
A SPAC I Acquisition (NASDAQ:ASCAU)
Gráfico Histórico do Ativo
De Nov 2024 até Dez 2024

A SPAC I Acquisition (NASDAQ:ASCAU)
Gráfico Histórico do Ativo
De Dez 2023 até Dez 2024
