false
0001574235
0001574235
2024-01-06
2024-01-06
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
8-K
CURRENT
REPORT
Pursuant
to Section 13 or 15(d) of the
Securities
Exchange Act of 1934
Date
of Report (Date of earliest event reported): January 6, 2024
PULMATRIX,
INC.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
001-36199 |
|
46-1821392 |
(State
or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(IRS
Employer
Identification No.) |
36
Crosby Drive, Suite 100
Bedford,
MA 01730
(Address
of principal executive offices) (Zip Code)
(781)
357-2333
(Registrant’s
telephone number, including area code)
N/A
(Former
name or former address, if changed since last report)
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under
any of the following provisions:
| ☐ |
Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ☐ |
Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 13e-4 (c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities
registered pursuant to Section 12(b) of the Act:
| Title
of each class |
|
Trading
Symbol(s) |
|
Name
of exchange on which registered |
| Common
Stock, par value $0.0001 per share |
|
PULM |
|
The
NASDAQ Stock Market LLC |
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405
of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ☐
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item
1.01 Entry into a Material Definitive Agreement.
As
previously disclosed, on April 15, 2019, Pulmatrix, Inc. (the “Company”) and Pulmatrix Operating Company, Inc. entered into
a Development and Commercialization Agreement (as amended on May 3, 2019 and November 8, 2021, the “Cipla Agreement”) with
Cipla Technologies LLC (“Cipla” and together with the Company and Pulmatrix Operating Company, Inc., the “Parties”),
for the co-development and commercialization, on a worldwide exclusive basis, of Pulmazole (PUR1900), the Company’s inhaled iSPERSE
drug delivery system (the “Product”) enabled formulation of the antifungal drug, itraconazole, for the treatment of all pulmonary
indications, including allergic bronchopulmonary aspergillosis in patients with asthma.
On
January 6, 2024 (the “Effective Date”), the Parties entered into the third amendment to the Cipla Agreement (the “Third
Amendment”), which terminates the Phase 2b clinical study (the “Phase 2b Clinical Study”) of the Product and makes
certain related modifications to the Cipla Agreement. The Third Amendment, among other things, provides that all development and commercialization
activities with respect to the Product in all countries outside the United States (such countries, the “Cipla Territory”)
will be conducted exclusively by Cipla, with all such development and commercialization expenses of the Product in the Cipla Territory
incurred after the Effective Date to be borne at Cipla’s sole cost and expense.
Additionally,
during the period commencing on the Effective Date and ending on July 30, 2024, the Company has agreed to use commercially reasonable
efforts to immediately close enrollment for the Phase 2b Clinical Study and to terminate the Phase 2b Clinical Study as soon as reasonably
possible. In connection with the termination of the Phase 2b Clinical Study (the “Development Wind Down Plan”), the Company
and Cipla will each be responsible for (i) 60% and 40%, respectively, of the Company’s overhead costs and the time spent by the
Company’s employees and consultants on development of the Product (the “Direct Costs”) associated with the Development
Wind Down Plan, and (ii) 50% each for all of the other development costs that are not Direct Costs (the “Non-Direct Costs”)
associated with the Development Wind Down Plan. The Company has agreed to work to immediately reduce all Non-Direct Costs to the minimum
amount necessary to terminate the Phase 2b Clinical Study, including through the termination of contract research organization sites
and other agreements, and to assign and/or transfer to Cipla all relevant contracts, regulatory documents, product data and other similar
documents required for the continued development of the Product, among others.
The
foregoing is only a summary of the material terms of the Third Amendment and does not purport to be complete. The foregoing summary is
qualified in its entirety by reference to the complete text of the Third Amendment, which is attached hereto as Exhibit 10.1 and incorporated
herein by reference.
Item
2.02 Results of Operations and Financial Condition.
On
January 8, 2024, the Company issued a press release (the “Press Release”) regarding its entry into the Third Amendment and
the termination of the Phase 2b Clinical Study, which is attached hereto as Exhibit 99.1. The Press Release additionally contains certain
preliminary selected financial information for the year ended December 31, 2023.
The
preliminary selected financial results reported by the Company are unaudited, subject to adjustment, and in accordance with General Instruction
B.2 of Form 8-K, being furnished pursuant to Item 2.02 and thus shall not be deemed to be “filed” for the purposes of Section
18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that
section, and shall not be incorporated by reference into any registration statement or other document filed under the Securities Act
of 1933, as amended (the “Securities Act”), or the Exchange Act, except as shall be expressly set forth by specific reference
in such filing.
Item
5.02 Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of
Certain Officers.
On
January 6, 2024, the Company and Teofilo Raad entered into a letter agreement (the “Letter Agreement”), pursuant to which
Mr. Raad shall be granted a retention bonus of $170,000 per quarter, for each of the full calendar quarters ending March 31, 2024 and
June 30, 2024, respectively, less applicable payroll and other tax withholdings (such bonus, the “Retention Bonus”). Pursuant
to the Letter Agreement, Mr. Raad must be employed by the Company on the last day of such applicable calendar quarter unless Mr. Raad’s
employment is terminated on the closing date of a potential acquisition of the Company by an unrelated third party, merger by the Company
with or into an unrelated third party or other liquidation event (such closing date, the “Retention Date”), in which case,
Mr. Raad shall receive the full Retention Bonus for the calendar quarter in which the Retention Date occurs.
Notwithstanding
the foregoing, if Mr. Raad’s employment with the Company is terminated by the Company without Cause (as defined in the Amended
and Restated Executive Employment Agreement by and between Mr. Raad and the Company dated June 28, 2019, as amended) prior to the Retention
Date, or due to death or disability, then the Company shall pay to Mr. Raad the full Retention Bonus with respect to the calendar quarter
of such termination, subject to the receipt of a release of claims by the Company (the “Release”). The Company shall not
be obligated to pay the Retention Bonus if (i) Mr. Raad terminates his employment with the Company prior to the Retention Date, (ii)
the Company terminates Mr. Raad’s employment prior to the Retention Date for Cause, or (iii) Mr. Raad’s employment is terminated
due to his death, disability or by the Company without Cause, and a Release has not been received.
The
foregoing is only a summary of the material terms of the Letter Agreement and does not purport to be complete. The foregoing summary
is qualified in its entirety by reference to the complete text of the Letter Agreement, which is attached hereto as Exhibit 10.2 and
incorporated herein by reference.
Item 7.01 Regulation FD Disclosure.
The Company also intends, from time to time, to
present and/or distribute to the investment community and utilize at various industry and other conferences a slide presentation, which
is attached hereto as Exhibit 99.2. The Company undertakes no obligation to update, supplement or amend the materials attached hereto
as Exhibit 99.2.
In accordance with General Instruction B.2 of
Form 8-K, the information in this Current Report on Form 8-K, including Exhibit 99.2, being furnished pursuant to Item 7.01, shall not
be deemed to be “filed” for the purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that
section, and shall not be incorporated by reference into any registration statement or other document filed under the Securities Act,
or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
Item
8.01 Other Events
On
January 8, 2024, the Company issued the Press Release, which is attached hereto as Exhibit 99.1 and incorporated herein by reference
(excluding the portions of Exhibit 99.1 that are furnished and not deemed “filed” pursuant to Item 2.02 of this Current Report
on Form 8-K).
Item
9.01 Financial Statements and Exhibits.
(d)
Exhibits
| Exhibit
No. |
|
Description |
| |
|
|
| 10.1* |
|
Third Amendment to Development and Commercialization Agreement, dated January 6, 2024, by and between the Company, Pulmatrix Operating Company, Inc., and Cipla Technologies LLC |
| 10.2 |
|
Letter Agreement, dated January 6, 2024, by and between Teofilo Raad and the Company |
| 99.1** |
|
Press Release, dated January 8, 2024 |
| 99.2 |
|
Investor Presentation (furnished
herewith pursuant to Item 7.01) |
| 104 |
|
Cover
Page Interactive Data File (embedded within the Inline XBRL document) |
*
Certain of the schedules (and similar attachments) to this exhibit have been omitted in accordance with Item 601(a)(5) of Regulation
S-K under the Securities Act because they do not contain information material to an investment or voting decision and that information
is not otherwise disclosed in the exhibit or the disclosure document. The registrant hereby agrees to furnish a copy of all omitted schedules
(or similar attachments) to the Securities and Exchange Commission upon its request.
**
Certain information within this exhibit is furnished pursuant to Item 2.02 and shall not be deemed to be “filed.”
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned hereunto duly authorized.
| |
PULMATRIX,
INC. |
| |
|
| Date:
January 8, 2024 |
By: |
/s/
Teofilo Raad |
| |
|
Teofilo
Raad |
| |
|
Chief
Executive Officer |
Exhibit
10.1
THIRD
AMENDMENT TO THE DEVELOPMENT AND COMMERCIALIZATION AGREEMENT
This
Third amendment to the Development and Commercialization Agreement (“Third
Amendment”) is entered into as of 6 January, 2024 (the “Effective Date”) and to the extent applicable amends
the Development and Commercialization Agreement entered into on April 15, 2019 (as amended by the parties on May 3, 2019 (the “First
Amendment”) and as further amended by the parties on November 8, 2021 (the “Second Amendment”) collectively
(the “Agreement”) between, PULMATRIX, INC. a company incorporated under the laws of the State of Delaware,
and PULMATRIX OPERATING COMPANY, INC., a company incorporated under the laws of the State of Delaware (together the “Company”),
and each having a principal place of business at 36 Crosby Drive, Suite 100, Bedford, Massachusetts 01730, and CIPLA TECHNOLOGIES,
LLC, a limited liability company organized under the laws of Delaware and having a principal place of business at 7 Oser Avenue,
Hauppauge, NY 11788 (“Cipla”). Company and Cipla may be referred to herein as a “Party” and collectively
as the “Parties.” Capitalized terms used herein but not otherwise defined herein shall have the respective meanings
given such terms in the Agreement.
RECITALS
Whereas:
| A. | Prior
to the date of this Third Amendment, the Parties initiated a new Phase 2b Clinical Study
of the Product (“Phase 2b Clinical Study.”) |
| B. | The
Parties agree to terminate the Phase 2b Clinical Study and consequently intend to suspend
further enrollment of patients in the Phase 2b Clinical Study and make certain related modifications
to the terms of the Agreement; and |
Now,
Therefore, in consideration of the foregoing premises
and the mutual covenants contained herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby
acknowledged, the Parties hereby agree as follows.
| 1.1 | Recitals
& Modifications. (a) The above preamble shall be an integral part of this Third
Amendment; (b) provisions of the Agreement not expressly modified or amended by this Third
Amendment shall continue to apply without any change thereto and (c) in the event of inconsistency
in the provisions of this Third Amendment and those of the Agreement, the provisions of this
Third Amendment shall prevail. |
| 1.2 | Amendments
to Article 1 of the Agreement. Article 1 of the Agreement is hereby amended by adding
the following definitions thereto in appropriate alphabetical order: |
“Third
Amendment” means the Third Amendment to the Agreement dated 6 January, 2024, by and between the Parties.
| 1.3 | Amendments
to Section 1.2 of the Second Amendment. |
| 1.3.1 | Section
1.2(a) of the Second Amendment is hereby amended and restated as follows with effect from
and after the Effective Date: |
“(a)
Subject to the terms of this Second Amendment, all Development and Commercialization activities with respect to the Product in all countries
outside of the United States (such countries outside of the United States, the “Cipla Territory”) will be conducted
exclusively by Cipla and all expenses associated with the development and commercialization of the Product in the Cipla Territory incurred
after the Effective Date, shall be at Cipla’s sole cost and expense. Accordingly, each reference to the Territory in the Agreement
is by reference amended to exclude the Cipla Territory, as the term has been defined in this Third Amendment. For the avoidance of doubt,
from the Effective date Cipla Territory shall mean and include all countries except the United States. ”
| 1.3.2 | Section
1.2(b) of the Second Amendment is hereby amended and restated as follows with effect from
and after the Effective Date: |
“(b)
All of Cipla’s Development activities for the Product in the Cipla Territory shall be conducted pursuant exclusively to one or
more plans (such plans, a “Cipla Territory Development Plan(s)”) prepared by Cipla.”
| 1.3.3 | Section
1.2(f) of the Second Amendment is hereby amended and restated as follows with effect from
and after the Effective Date: |
“(f)
All Product used in the Development or Commercialization in the Cipla Territory may at Cipla’s decision and sole cost be supplied
utilizing the Company’s existing global supply chain for manufacturing of the Product (it being understood that Cipla will need
to enter new agreements with the Company’s existing vendors and suppliers in order to utilize such supply chain). Cipla, may, at
its option and at its sole cost and expense, transfer the manufacturing of the Product for Development or Commercialization activities
in the Cipla Territory to a manufacturing site designated by Cipla. Any such manufacturing site shall be qualified under applicable laws
and such site transfer shall be conducted in accordance with applicable laws and regulations, good manufacturing practices (as defined
by the International Council on Harmonization). The Company shall cooperate with Cipla in such site transfer and all actual
costs and expenses, including cost of time spent by the Company’s employees and consultants, incurred by the Company in connection
with such site transfer to the extent that such cost is solely incurred with respect to the Cipla Territory shall be paid by Cipla in
the Wind Down Phase Funding contributed by Cipla.”
| 1.3.4 | Section
1.2(g) of the Second Amendment is hereby amended and restated as follows with effect from
and after the Effective Date: |
“(g)
Section 6.5 of the Agreement shall not apply with respect to any sublicense or other transfer of Cipla’s rights under the
Agreement and this Second Amendment with respect to the Cipla Territory. For clarity, (i) nothing herein impacts Cipla’s ownership
of the Assigned Assets as detailed under Section 6.1 of the Agreement; and (ii) Cipla will solely and exclusively own all of the Intellectual Property Rights generated with respect to the Cipla Territory, provided, that, without limiting Cipla’s absolute ownership of Assigned
Assets and the Cipla Territory Intellectual Property Rights as stated in the foregoing sentence.”
| 1.4 | Amended
Phase 2b Development Plan. |
| 1.4.1 | The
Parties previously adopted a Development Plan, including a budget of all Development Costs,
for a Phase 2b Clinical Study of the Product for the treatment of ABPA (the “Phase
2b Development Plan”). |
| 1.4.2 | During
the period commencing on the Effective Date and ending July 30, 2024 (the “Wind
Down Period”); the Parties agree to do the following, at no extra cost to Cipla,
except as otherwise approved in a JSC approved Budget and subject to appropriate proportion
of expenses sharing between the Company and Cipla as stated below: |
1.4.2.1.1 the
Company will use Commercially Reasonable Efforts to immediately close enrollment for the Phase 2b Clinical Study and terminate the Phase
2b Clinical Study as soon as reasonably possible;
1.4.2.1.2 Exhibit
C sets forth the patents related to the Product and Cipla’s rights therein as previously licensed and assigned pursuant to Article
6 of the Agreement. The Company will reasonably cooperate with Cipla in perfecting such assignment or license of all Patents (as in Exhibit
C) to Cipla and their registration with the appropriate authorities in the Cipla Territory;
1.4.2.1.3 reserved;
1.4.2.1.4 the
Company will to the extent not yet completed, complete a physical and demonstrable Company assisted technology transfer (“Tech
Transfer”) and assignment of all Development, Intellectual Property Rights, proprietary data and technology (“Proprietary
Data and Technology”) and technology to Cipla, an indicative and non-exhaustive list of which activity items is attached as
EXHIBIT A; this list being subject to further changes and supplementation by Cipla and the Company;
1.4.2.1.5 during
the Wind Down Period, each Party will use Commercially Reasonable Efforts to secure all data from the Phase 2b Clinical Study for inclusion
in the safety database for the Cipla Territory program;
1.4.2.1.6 a
development wind down plan is attached hereto as Exhibit B (“the Development Wind Down Plan”) for implementation and
closure within the Wind Down Period, for approval by the JSC, the Development Wind Down Plan being effective immediately upon such approval;
1.4.2.1.7 all
Direct Costs to terminate the Phase 2b Clinical Study shall be within the budget approved in Development Wind Down Plan and the Company
and Cipla will each be responsible for sixty percent (60%) and forty percent (40%), respectively of Wind Down Period Direct Costs;
1.4.2.1.8
the Company will work to immediately reduce all Non-Direct Costs to the minimum amount necessary to terminate the Phase 2b Clinical
Study, including the termination of CRO or contract research organization, sites and other agreements and within the budget approved
in Development Wind Down Plan (allowing for possible eight percent (8%) overage from the budget), the Company and Cipla then being
responsible for the Wind Down Period Non-Direct Costs in the manner set out in Clause 3.5 of the Agreement as amended by Clause 1.5.1 of this Amendment;
1.4.2.1.9 Cipla
shall pay Company for the period up to the Effective Date, all accrued Direct and Non-Direct Costs computed according to the Second Amendment;
1.4.2.2 Exhibit
B is the Company expected timeline for Phase 2b clinical study wind down;
1.4.2.2.1 all
relevant contracts, regulatory documents, product data and other similar documents required for the continued development of the Product
shall be assigned and transferred to Cipla during the Wind Down Period; and
1.4.2.2.2 the
Parties’ shall use Commercially Reasonable Efforts to monetize the Parties’ rights in the Product within the United States.
| 1.4.2.3 | The
Parties understand that expenses incurred during the Wind Down Period may not be invoiced
by third party vendors until after the expiration of the Wind Down Period and the Parties
shall remain liable for the respective share of any expenses appropriately incurred during
the Wind Down Period regardless of when invoiced. |
| | | |
| 1.4.2.4 | In
addition to the requirements stated in this Amendment, Cipla and the Company representatives
may add other reasonable items to the list of requirements including but not limted to intellectual
property, iSPERSETM for Product, data and technology transfer. |
| 1.5 | Amendment
to Section 3.5. |
| 1.5.1 | Section
3.5 of the Agreement is hereby amended and restated as follows with effect from and after
the date hereof: |
“
3.5 Winding Down Period Contributions.
Winding
Down Phase. After the Initial Development Funding is depleted, Company shall, within ten (10) days after the end of each calendar
month, deliver an invoice to Cipla for the Winding Down Period costs incurred by the Company, including a breakdown of Direct Costs and
Non-Direct Costs, for such calendar month and for which Cipla is responsible pursuant to Section 3.5(a). By not later than the last Business
Day of the calendar month in which such invoice was delivered to Cipla, Cipla shall deliver to the Company, by wire transfer of immediately
available funds, the invoiced amount. Attached as EXHIBIT-D is the proposed Wind Down Budget provided by the Company to Cipla, to be
discussed, negotiatited, finalized and approved by the JSC. The parties agree that Cipla shall not be responsible for any Non-Direct
Cost that is in excess of the budgeted amount therefor set forth on EXHIBIT-D by more than 8%. Furthermore, the parties agree that that
Cipla shall not be responsible for any Direct Cost that is in excess of the budgeted amount therefor set forth on EXHIBIT-D.
| 1.6 | Amendment
to Section 12.4. |
Section
12.4 of the Agreement is hereby amended and restated as follows with effect from and after the date hereof:
| 1.6.1 | Company
Termination. Upon written notice to Cipla, the Company shall be entitled to terminate
the Agreement upon a sale of all or substantially all assets of the Company or a merger or
consolidation involving the Company. For clarity, any Intellectual Property licenses contained
in the Agreement that are necessary or Cipla to continue the Development and Commercialization
of the Product in the Cipla Territory shall survive any termination of this Agreement pursuant
to this Section 12.4. |
| 1.6.2 | In
the event that Company desires to abandon or cease prosecution or maintenance of any licensed
patents, and trade secrets in any country or in the event of the Company’s bankruptcy
is unable to so prosecute or maintain, the Company (or its successor or as applicable its
bankruptcy administrator or trustee) shall
provide reasonable prior written notice to Cipla of such intention to abandon or cease prosecution
(which notice shall, to the extent possible, be given no later than 60 days prior to the
next deadline for any action that must be taken with respect to any such patents in the relevant
patent office). In such case, at Cipla’s sole discretion, upon written notice to the
Company (or its successor or as applicable its bankruptcy administrator or
trustee)), Cipla may elect to assume responsibility for prosecution and maintenance
of such patents, at Cipla’s sole cost and expense and by counsel of its own choice,
in which event the Company (or its successor or as applicable its bankruptcy administrator
or trustee) shall at no cost assign the respective
patents to Cipla. |
| 1.6.3 | Intellectual
Property Rights Protection. The Agreement is the intellectual property licensing agreement
for the applicable Intellectual Property Rights and licensed technology, including for the
purposes of the United States Bankruptcy Code §365(n) protection of Cipla’s continued
use of the licensed Intellectual Property Rights and licensed technology, in the event of
the Company’s bankruptcy. |
| 1.6.4 | For
clarity, the provisions of clauses 1.6.2 and 1.6.3 above shall apply to the iSPERSETM
technology. |
| 1.6.5 | Entire
Agreement. This Third Amendment, as part of the Agreement, constitutes the entire
agreement between the Parties with respect to the subject matter herein, and supersedes all
prior agreements, proposals, negotiations, representations or communications relating to
such subject matter. The Parties acknowledge that they have not been induced to enter into
this Agreement by any representations or promises not specifically stated herein. |
| 1.6.6 | Execution;
Counterparts. This Third Amendment may be executed in multiple counterparts, each
of which shall be deemed an original, but all of which together shall constitute one and
the same instrument. Facsimile signatures, including signatures in a fixed electronic format
such as PDF, shall have the same effect as originals. |
(Signature
Page Follows)
IN
WITNESS WHEREOF, THE PARTIES HAVE EXECUTED THIS THIRD AMENDMENT AS OF THE EFFECTIVE DATE.
| PULMATRIX,
INC. |
|
PULMATRIX
OPERATING COMPANY, INC. |
| |
|
|
| By: |
/s/ Teofilo
Raad |
|
By: |
/s/ Teofilo
Raad |
| Name: |
Teofilo Raad |
|
Name: |
Teofilo Raad |
| Title: |
Chief
Executive Officer
|
|
Title: |
Chief
Executive Officer |
| Date: |
January 6, 2023 |
|
Date: |
January
6, 2023 |
| |
|
|
|
|
| Cipla
Technologies LLC |
|
|
|
| |
|
|
|
| By: |
/s/
Dr. Jaideep Gogtay |
|
|
|
| Name: |
Dr.
Jaideep Gogtay |
|
|
|
| Title: |
EVP
and Chief Medical Officer |
|
|
|
| Date: |
January 5, 2024 |
|
|
|
Exhibit
10.2
| To: |
Teofilo
Raad |
| |
|
| From: |
Pulmatrix,
Inc. |
| |
|
| Date: |
January
6, 2024 |
| |
|
| Subject: |
Retention
Bonus Opportunity |
Pulmatrix,
Inc. (the “Company”) is in a period of transition. You are a valued member of our team, and we need you to
help the Company meet the challenges ahead. To recognize your service to the Company, and to retain your ongoing and future employment
with the Company, we are pleased to present you with a retention bonus opportunity, subject to the terms and conditions set forth below.
Terms capitalized but not otherwise defined herein shall have the meaning given to such terms in that certain Amended and Restated Executive
Employment Agreement by and between you and the Company dated June 28, 2019, as amended (the “Employment Agreement”).
We
appreciate your commitment to the Company. To accept this retention bonus opportunity, please sign, date and return this letter.
TERMS
AND CONDITIONS
| a. | You
agree to remain employed with the Company through the closing date of a potential acquisition
of the Company by an unrelated third party, merger by the Company with or into an unrelated
third party or other liquidation event (the “Retention Date”).
As consideration for your agreement, the Company agrees to pay you a retention bonus for
the full calendar quarters ending March 31, 2024 and June 30, 2024, respectively, provided
you remain employed during such quarters, equal to $170,000 each quarter (each “Retention
Bonus”), less all applicable payroll and other tax withholdings, payable on
the first payroll period of the month following the calendar quarter to which the Retention
Bonus relates (for example, the Retention Bonus for the quarter ending March 31, 2024 will
be paid in April of 2024). Except as otherwise provided by Section 1.b. below, you must be
employed on the last day of the applicable calendar quarter to receive the Retention Bonus
for such calendar quarter unless your employment is terminated on the Retention Date, in
which case, you shall receive the full Retention Bonus for the calendar quarter in which
the Retention Date occurs. |
| b. | Notwithstanding
the foregoing, if your employment with the Company is terminated prior to the Retention Date
by the Company without Cause (as defined in the Employment Agreement) or due to your death
or Disability (as defined in the Employment Agreement), then the Company shall pay the full
Retention Bonus with respect to the calendar quarter of your termination of employment to
you (or to your estate) on the Company’s next regularly scheduled payroll date following
the date you (or your estate or legal representative) return a validly executed, irrevocable
release of claims in the form provided by the Company at the time of your termination (the
“Release”) and such Release becomes effective; provided, however,
that in the event the time period for signing the Release, plus the expiration of any applicable
revocation period, begins in one taxable year and ends in a second taxable year, payment
of the Retention Bonus will not be made until the second taxable year. |
| c. | The
Company shall have no obligation to pay you the Retention Bonus for the month of your termination
of employment if (i) you terminate your employment with the Company prior to the Retention
Date, (ii) the Company terminates your employment with Cause prior to the Retention Date,
or (iii) your employment terminates prior to the Retention Date due to your death or Disability
or the Company’s termination of your employment without Cause and you (or your estate
or legal representative) refuse to sign the Release (or revoke the Release). |
| 2. | Right
to Continued Employment. Please note that your eligibility for the Retention Bonus does
not in any way alter, modify, or amend your relationship with the Company, nor does it guarantee
you the right to continue in the employ or service of the Company. |
| 3. | Other
Benefits. The Retention Bonus is a special incentive payment to you and will not be taken
into account in computing the amount of salary or compensation for purposes of determining
any bonus, incentive, severance, pension, retirement, death or other benefit under any other
bonus, incentive, pension, retirement, insurance, or other employee benefit plan of the Company,
unless such plan or agreement expressly provides otherwise. |
| 4. | Governing
Law. All questions concerning the construction, validity, and interpretation of this
letter will be governed by the laws of the State of Massachusetts, without giving effect
to conflict of laws principles thereof. |
| 5. | Entire
Agreement. This letter constitutes the entire agreement between you and the Company with
respect to the Retention Bonus and supersedes any and all prior agreements or understandings
between you and the Company with respect to the Retention Bonus, whether written or oral.
This letter may be amended or modified only by a written instrument executed by you and the
Company. |
We
ask that you acknowledge your receipt of this letter and your acceptance of its terms and conditions by signing and dating the Acknowledgement
and Acceptance section below and returning it to me for the Company’s records by the Acceptance Date.
Very
truly yours,
| /s/
Michael J. Higgins |
|
| Michael
J. Higgins, Chairman |
|
ACKNOWLEDGEMENT
AND ACCEPTANCE
I
hereby acknowledge receipt of this letter setting forth the terms and conditions governing the opportunity to receive the Retention Bonuses.
I have carefully read the letter and hereby agree to and accept all those terms and conditions, and agree that my entitlement to any
Retention Bonus described in the letter shall be determined solely by the terms and conditions described herein.
| /s/
Teofilo Raad |
|
| Signature
|
|
| |
|
| Printed
Name: Teofilo Raad |
|
| Dated:
January 6, 2024 |
|
Exhibit
99.1

Pulmatrix
Announces Stopping the PUR1900 Phase 2B Study Patient Enrollment and Closing the Study, in Agreement With Partner Cipla, to Preserve
Cash and Facilitate Pursuit of Strategic Alternatives
Cipla
to take sole responsibility for development of PUR1900, refocused on markets with greatest unmet need and faster path to approval, in
exchange for 2% royalty on net sales payable to Pulmatrix
Pulmatrix
to significantly reduce cash burn and focus on strategic alternatives that leverage the company’s promising pipeline, iSPERSE™
technology and approximately $19 million cash on hand as of 12/31/23
BEDFORD,
Mass., January 8th, 2024 – Pulmatrix, Inc. (“Pulmatrix” or the “Company”) (NASDAQ: PULM),
a clinical-stage biopharmaceutical company developing innovative inhaled therapies to address serious pulmonary and central nervous system
diseases, today announced that it has entered into a further amendment to its agreement with Cipla for the development of PUR1900 in
the treatment of Allergic Bronchopulmonary Aspergillosis (ABPA). Under the terms of the amendment Cipla and Pulmatrix agreed, among other
things, to stop patient enrollment of the Ph2b study of PUR1900 at 8 subjects and close the study.
Ted
Raad, Chief Executive Officer of Pulmatrix, commented, “Stopping the Ph2b study, along with other cost-savings measures, is expected
to extend Pulmatrix’s cash runway into Q1 2026. As of December 31, 2023, Pulmatrix cash on hand was approximately nineteen million
dollars. Pulmatrix focus will be on maximizing shareholder value by pursuing strategic alternatives for the company while it winds down
the Ph2b study for PUR1900. We are confident in Cipla’s ability to develop PUR1900 for the benefit of patients in markets where
there is significant unmet need and a faster path to commercialization.”
Pursuant
to the amended agreement with Cipla, Pulmatrix has granted Cipla exclusive rights to the development and commercialization of PUR1900
in the “Cipla Territory”, which has been expanded to include all markets other than the United States. In the United States,
both parties will seek to monetize PUR1900 which has potential for development in areas other than ABPA in Asthma. After the study winddown,
Pulmatrix will bear no further financial responsibility for the development of PUR1900 and will receive 2% royalties on net sales of
Pulmazole in the Cipla Territory. For more information about the amendment please refer to Pulmatrix’s Current Report on Form 8-K
to be filed with U.S. Securities and Exchange Commission on or around the date of this press release.
Pulmatrix
has retained MTS Health Partners, L.P. (MTS) as its financial advisor to support the company’s Board of Directors and management
team to review and evaluate strategic alternatives intended to maximize long-term value for all stockholders.
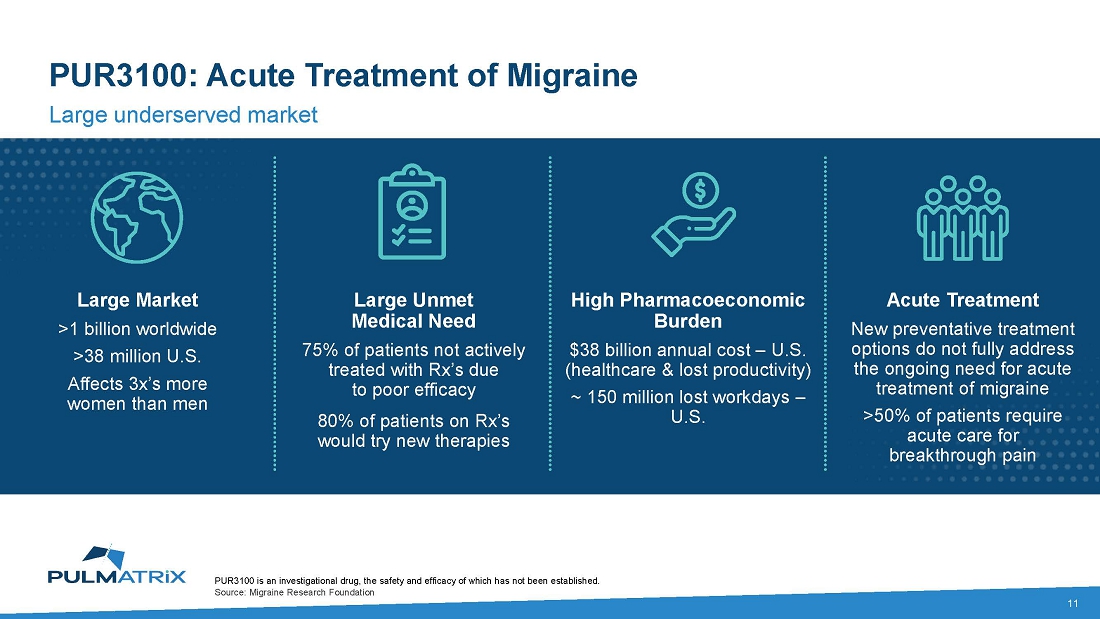
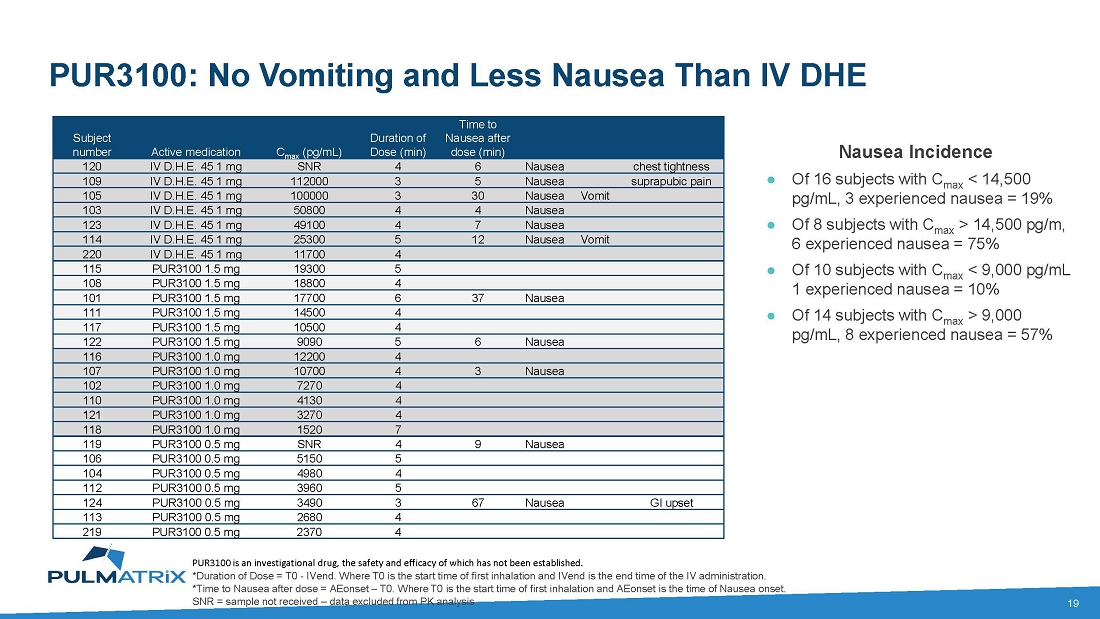
About
PUR3100
PUR3100
is an orally inhaled formulation of dihydroergotamine (DHE) engineered with iSPERSE™ for the treatment of acute migraine. The Phase
1 PUR3100 trial results were presented at the 65th Annual Meeting of the American Headache society in June 2023. The PUR3100 IND for
a Phase 2 trial was accepted by the FDA in September 2023.

About
PUR1800
PUR1800
is an orally inhaled formulation of RV1162, a narrow-spectrum kinase inhibitor with p38, Syc and Src kinase activity. PUR1800 is being
developed for the treatment of acute exacerbations in Chronic Obstructive Pulmonary Disease. The Phase 1 PUR1800 trial results were presented
at the Annual Meeting of the American Academy of Allergy, Asthma and Immunology in February 2023.
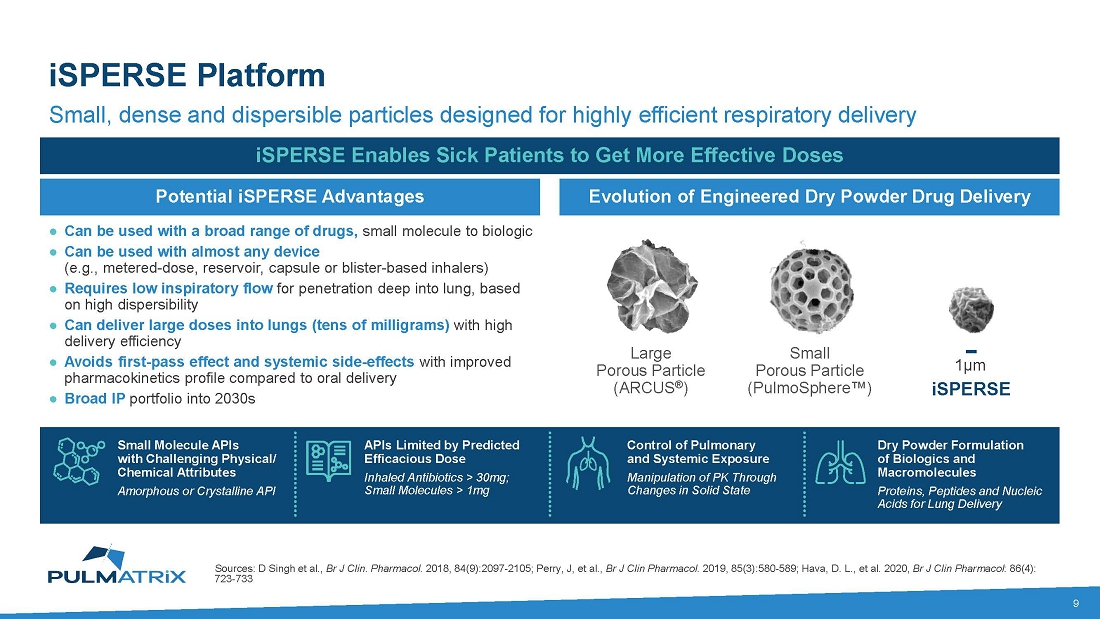
About
iSPERSE™ Technology
Our
innovative particle engineering technology creates dry powder, which solves limitations of conventional inhaled technologies and expands
the universe of inhalable drug therapies. iSPERSE is a proprietary technology that allows a broad range of drugs to be formulated as
small, dense, and dispersible particles for highly efficient drug delivery and deep penetration into the lungs. iSPERSE can efficiently
deliver small molecules, drug combinations, peptides, proteins, and nucleic acids via the respiratory system for the treatment of both
respiratory and non-respiratory diseases.
About
Pulmatrix, Inc.
Pulmatrix
is a clinical-stage biopharmaceutical company developing innovative inhaled therapies to address serious pulmonary diseases and central
nervous system (“CNS”) disorders using its patented iSPERSE™ technology. The Company’s proprietary product pipeline
includes treatments for lung diseases, such as allergic bronchopulmonary aspergillosis (“ABPA”), Chronic Obstructive Pulmonary
Disease (“COPD”) and CNS disorders such as acute migraine. Pulmatrix’s product candidates are based on its proprietary
engineered dry powder delivery platform, iSPERSE™, which seeks to improve therapeutic delivery to the lungs by maximizing local
concentrations and reducing systemic side effects to improve patient outcomes.
For
more on our inhaled product candidates please visit: https://www.pulmatrix.com/pipeline.html.
Forward-Looking
Statements
Certain
statements in this press release that are forward-looking and not statements of historical fact are forward-looking statements within
the meaning of the federal securities laws. Such forward-looking statements include, but are not limited to, statements of historical
fact and may be identified by words such as “anticipates,” “assumes,” “believes,” “can,”
“could,” “estimates,” “expects,” “forecasts,” “guides,” “intends,”
“is confident that”, “may,” “plans,” “seeks,” “projects,” “targets,”
and “would,” and their opposites and similar expressions are intended to identify forward-looking statements. Such forward-looking
statements are based on the beliefs of management as well as assumptions made by, and information currently available to management.
Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, including,
but not limited to, the impact of the novel coronavirus (COVID-19) on the Company’s ongoing and planned clinical trials; realizing
the expected cost savings from closing the Ph2b study of PUR1900, the ability to identify and consummate strategic alternatives for the
Company, our ability to preserve and allocate our cash on-hand most efficiently, the geographic, social and economic impact of COVID-19
on the Company’s ability to conduct its business and raise capital in the future when needed; delays in planned clinical trials;
the ability to establish that potential products are efficacious or safe in preclinical or clinical trials; the ability to establish
or maintain collaborations on the development of therapeutic candidates; the ability to obtain appropriate or necessary governmental
approvals to market potential products; the ability to obtain future funding for developmental products and working capital and to obtain
such funding on commercially reasonable terms; the Company’s ability to manufacture product candidates on a commercial scale or
in collaborations with third parties; changes in the size and nature of competitors; the ability to retain key executives and scientists;
the ability to secure and enforce legal rights related to the Company’s products, including patent protection. A discussion of
these and other factors, including risks and uncertainties with respect to the Company, is set forth in the Company’s filings with
the Securities and Exchange Commission, including its most recent Annual Report on Form 10-K, as may be supplemented or amended by the
Company’s Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. The Company disclaims any intention or obligation to
revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Investor
Contact:
Timothy
McCarthy, CFA
917-679-9282
tim@lifesciadvisors.com
Exhibit
99.2



























v3.23.4
Cover
|
Jan. 06, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 06, 2024
|
| Entity File Number |
001-36199
|
| Entity Registrant Name |
PULMATRIX,
INC.
|
| Entity Central Index Key |
0001574235
|
| Entity Tax Identification Number |
46-1821392
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
36
Crosby Drive
|
| Entity Address, Address Line Two |
Suite 100
|
| Entity Address, City or Town |
Bedford
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
01730
|
| City Area Code |
(781)
|
| Local Phone Number |
357-2333
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common
Stock, par value $0.0001 per share
|
| Trading Symbol |
PULM
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Pulmatrix (NASDAQ:PULM)
Gráfico Histórico do Ativo
De Mar 2024 até Abr 2024
Pulmatrix (NASDAQ:PULM)
Gráfico Histórico do Ativo
De Abr 2023 até Abr 2024