false000131410200013141022024-05-282024-05-28
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported) May 28, 2024 |
EyePoint Pharmaceuticals, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
000-51122 |
26-2774444 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
480 Pleasant Street |
|
Watertown, Massachusetts |
|
02472 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (617) 926-5000 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.001 |
|
EYPT |
|
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
On May 28, 2024, EyePoint Pharmaceuticals, Inc. posted an updated investor presentation on its website at www.eyepointpharma.com. A copy of the presentation is filed herewith as Exhibit 99.1 and is incorporated by reference herein.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
EYEPOINT PHARMACEUTICALS, INC. |
|
|
|
|
Date: |
May 28, 2024 |
By: |
/s/ George O. Elston |
|
|
|
George O. Elston
Executive Vice President and Chief Financial Officer |

Investor Presentation May 2024 ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Exhibit 99.1

Legal Disclaimers ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Various statements made in this presentation are forward-looking, within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, and are inherently subject to risks, uncertainties and potentially inaccurate assumptions. All statements that address activities, events or developments that we intend, expect, plan or believe may occur in the future, including but not limited to statements about the sufficiency of our existing cash resources through topline data for Phase 3 clinical trials for DURAVYU™ in wet AMD; our expectations regarding the timing and clinical development of our product candidates, including DURAVYU and EYP-2301; the potential for DURAVYU as a novel sustained delivery treatment for serious eye diseases, including wet age-related macular degeneration, non-proliferative diabetic retinopathy and diabetic macular edema; and our longer term financial and business goals and expectations, are forward-looking statements. Some of the factors that could cause actual results to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements are risks and uncertainties inherent in our business including, without limitation: the effectiveness and timeliness of clinical trials, and the usefulness of the data; the timeliness of regulatory approvals; our ability to access needed capital; termination or breach of current and future license agreements; our dependence on contract research organizations, co-promotion partners, and other outside vendors and service providers; effects of guidelines, recommendations and studies; protection of our intellectual property and avoiding intellectual property infringement; retention of key personnel; product liability; industry consolidation; compliance with environmental laws; manufacturing risks; risks and costs of international business operations; volatility of our stock price; possible dilution; absence of dividends; the impact of instability in general business and economic conditions, including changes in inflation, interest rates and the labor market; and other factors described in our filings with the Securities and Exchange Commission. We cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized. A variety of factors, including these risks, could cause our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements. Our forward-looking statements speak only as of the dates on which they are made. We do not undertake any obligation to publicly update or revise our forward-looking statements even if experience or future changes makes it clear that any projected results expressed or implied in such statements will not be realized.
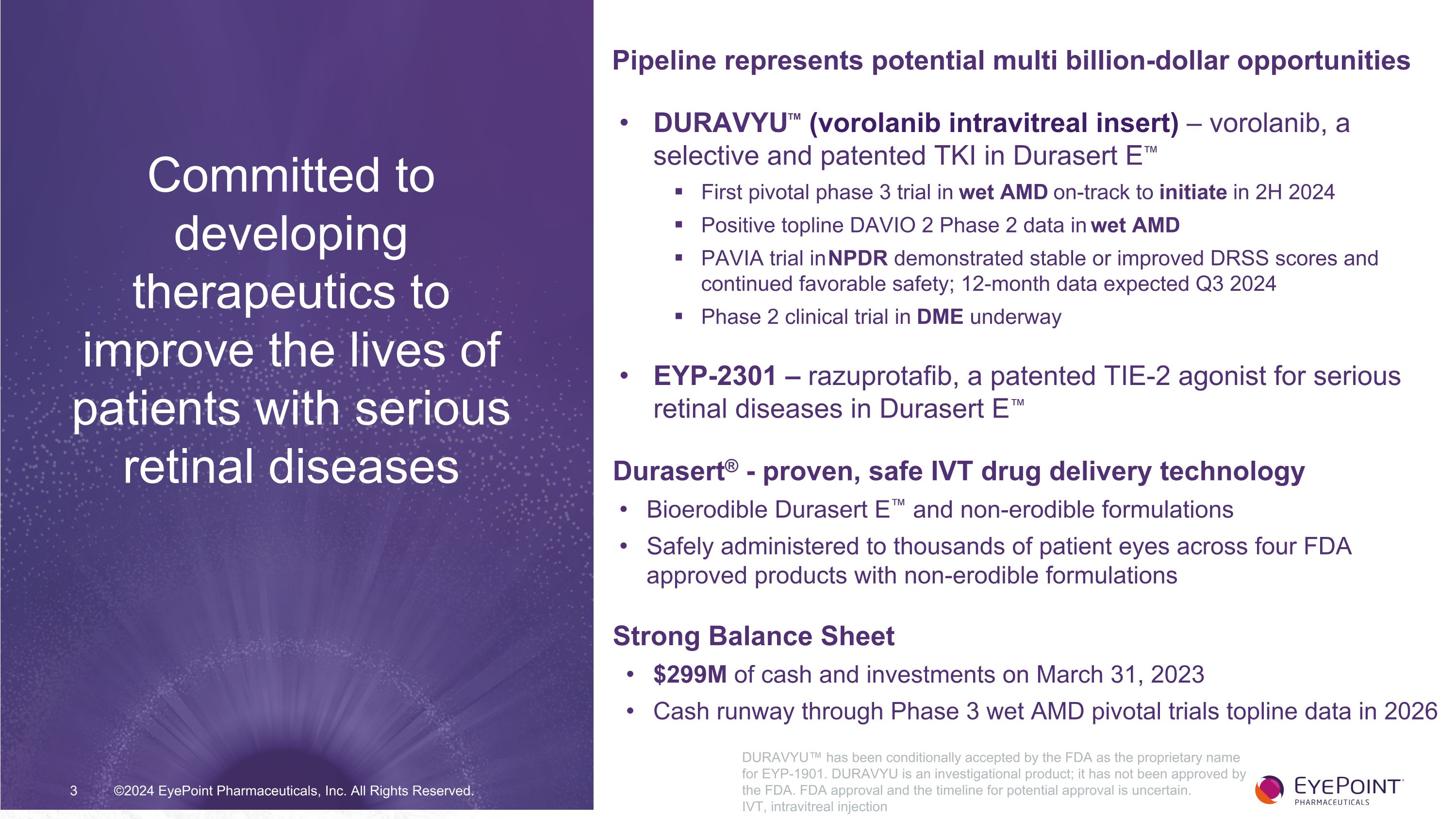
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Pipeline represents potential multi billion-dollar opportunities DURAVYU™ (vorolanib intravitreal insert) – vorolanib, a selective and patented TKI in Durasert E™ First pivotal phase 3 trial in wet AMD on-track to initiate in 2H 2024 Positive topline DAVIO 2 Phase 2 data in wet AMD PAVIA trial in NPDR demonstrated stable or improved DRSS scores and continued favorable safety; 12-month data expected Q3 2024 Phase 2 clinical trial in DME underway EYP-2301 – razuprotafib, a patented TIE-2 agonist for serious retinal diseases in Durasert E™ Durasert® - proven, safe IVT drug delivery technology Bioerodible Durasert E™ and non-erodible formulations Safely administered to thousands of patient eyes across four FDA approved products with non-erodible formulations Strong Balance Sheet $299M of cash and investments on March 31, 2023 Cash runway through Phase 3 wet AMD pivotal trials topline data in 2026 Committed to developing therapeutics to improve the lives of patients with serious retinal diseases DURAVYU™ has been conditionally accepted by the FDA as the proprietary name for EYP-1901. DURAVYU is an investigational product; it has not been approved by the FDA. FDA approval and the timeline for potential approval is uncertain. IVT, intravitreal injection

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. wet AMD, wet age-related macular degeneration; EOP2, End of Phase 2; FPI, first patient in; NPDR, non-proliferative diabetic retinopathy; DME, diabetic macular edema; GA, geographic atrophy Durasert E™ Programs Indication Discovery Pre-Clin Phase 1 Phase 2 Phase 3 Next Milestone DURAVYU (EYP-1901) – vorolanib in Durasert E™ (tyrosine kinase inhibitor) Wet AMD NPDR DME EYP-2301 – razuprotafib in Durasert E™ (TIE-2 agonist) serious retinal diseases Complement inhibition GA First Phase 3 Trial 2H 2024 12-month data Q3 2024 Topline data in Q1 2025 Pre-clin tox and PK data Potential product candidate in 2024 trial underway non-clinical Potential Multi Billion-Dollar Product Opportunities Leveraging Innovative Drug Delivery Technology, Bioerodible Durasert E™

Safe, Sustained IVT Drug Delivery Delivered via a standard in-office IVT injection Continuous, stable release of drug Zero-order kinetics Durasert®: non-erodible Drug embedded within a bioerodible matrix covered with non-erodible polyimide shell: YUTIQ®1 ILUVIEN®1 RETISERT®2 VITRASERT®2 Durasert E™: bioerodible Drug embedded within a bioerodible matrix No polyimide shell Designed to deplete drug load before matrix fully erodes DURAVYU™ TECHNOLOGY�DURASERT® 1- licensed to Alimera; 2 – licensed to Bausch and Lomb Durasert - Intravitreal Sustained-Release Drug Delivery ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved.

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 1. Sophie Bakri, M.D., et al. PLOS ONE, Vorolanib, sunitinib, and axitinib: A comparative study of vascular endothelial growth factor receptor inhibitors and their anti-angiogenic effects, 2024. VEGF(R), vascular endothelial growth factor (receptor); PDGF(R), platelet-derived growth factor (receptor); TIE-2, tyrosine-protein kinase receptor Potent and selective pan–VEGF receptor inhibition Composition of matter patent into 2037 Demonstrated neuroprotection in a validated retinal detachment animal model Inhibits PDGF which may lead to antifibrotic benefit Reduced off-target binding - does not inhibit TIE-2 at clinically relevant doses1 Vorolanib Brings a Potential New MOA to the Treatment of VEGF-Mediated Retinal Diseases by Inhibiting all Isoforms of VEGF and PDGF

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Insert is ~1/5000 �of vitreous volume Positive efficacy data in wet AMD from Phase 1 DAVIO and Phase 2 DAVIO 2 clinical trials Favorable safety profile with no ocular or systemic DURAVYU-related SAEs reported in ongoing Phase 2 clinical trials Immediately bioavailable featuring an initial burst of drug followed by zero order kinetics release Vorolanib fully eluted prior to complete bioerosion of the matrix to control release and allow redosing regimen Delivered in the physician office via routine intravitreal injection Shipped and stored at ambient temperature DURAVYU: VEGF Receptor Binding Vorolanib In Bioerodible Durasert E™ VEGF – vascular endothelial growth factor: AMD – age related macular degeneration;

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Phase 2 DAVIO 2 Clinical Trial Topline Results in wet AMD A NON-INFERIORITY TRIAL VERSUS AN AFLIBERCEPT CONTROL

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. The DAVIO 2 Clinical Trial in wet AMD A non-inferiority trial evaluating two doses of DURAVYU against an aflibercept control as a potential 6-month maintenance therapy Design: Multi-center, randomized, double-masked trial in patients with previously treated wet AMD Primary outcome: Difference in mean change in BCVA from Day 1 to Week 28 and 32 (blended) Key secondary endpoints: Safety Reduction in treatment burden Percent of eyes supplement-free up to six months Anatomical results Anti-VEGF supplement criteria: 5 letter loss with 75 microns of new fluid 10 letter loss due to wet AMD 100 microns new fluid x 2 visits New retinal hemorrhage from wet AMD Investigator discretion
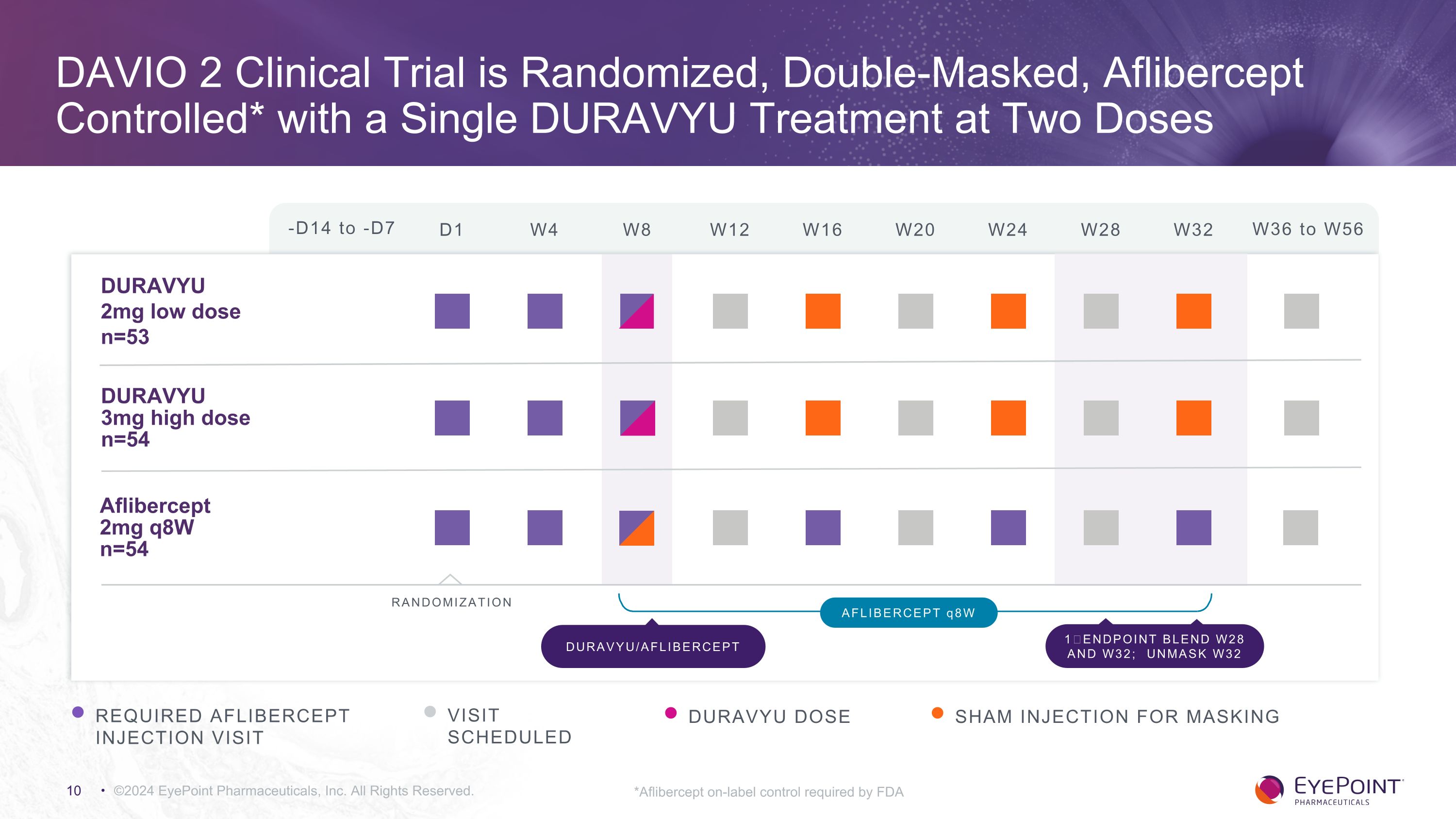
DAVIO 2 Clinical Trial is Randomized, Double-Masked, Aflibercept Controlled* with a Single DURAVYU Treatment at Two Doses ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. -D14 to -D7 D1 W4 W8 W12 W16 W24 W32 W36 to W56 W20 W28 DURAVYU �2mg low dose n=53 DURAVYU �3mg high dose n=54 Aflibercept 2mg q8W n=54 RANDOMIZATION REQUIRED AFLIBERCEPT INJECTION VISIT VISIT SCHEDULED DURAVYU DOSE AFLIBERCEPT q8W DURAVYU/AFLIBERCEPT 1⁰ ENDPOINT BLEND W28 AND W32; UNMASK W32 SHAM INJECTION FOR MASKING *Aflibercept on-label control required by FDA

DAVIO 2 Patient Baseline Characteristics Well Balanced Across Arms ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Aflibercept 2mg q8W (n=54) DURAVYU 2mg (n=50) DURAVYU 3mg (n=52) Mean age, years (range) 75.9 (52-93) 76.4 (61-93) 75.4 (56-89) Female, % 53.7% 64.0% 67.3% Mean BCVA, ETDRS letters (range) 73.4 (41-85) 73.9 (52-84) 74.9 (46-85) Mean CST, μm (range) 265.7 (178-348) 267.0 (192-400) 262.9 (186-345) Median length of time for wet AMD diagnosis prior to screening, months (range) 28.1 (2.4-273.8) 24.3 (2.4-168.1) 28.1 (2.4-145.3) Mean # of injections normalized to 12 months prior to screening (range)* 9.5 (2-12) 10.2 (2-13) 10.0 (2-13) PRELIMINARY DATA – PENDING FINAL ANALYSIS�AMD, age-related macular degeneration; BCVA, best-corrected visual acuity; CST, central subfield thickness; ETDRS, Early Treatment Diabetic Retinopathy Study; VEGF, vascular endothelial growth factor. Heavily pre-treated group
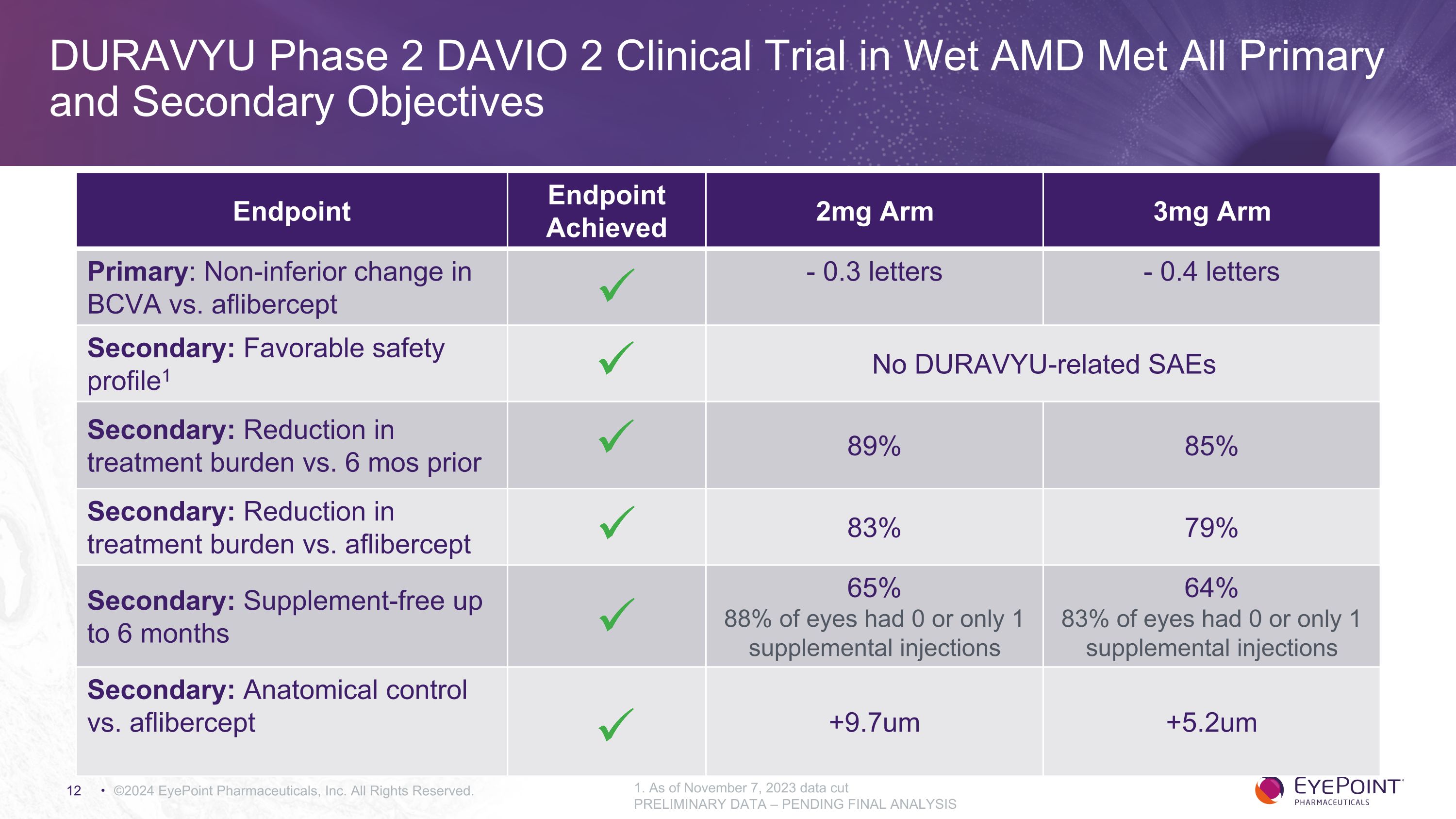
DURAVYU Phase 2 DAVIO 2 Clinical Trial in Wet AMD Met All Primary and Secondary Objectives ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 1. As of November 7, 2023 data cut PRELIMINARY DATA – PENDING FINAL ANALYSIS Endpoint Endpoint Achieved 2mg Arm 3mg Arm Primary: Non-inferior change in BCVA vs. aflibercept - 0.3 letters - 0.4 letters Secondary: Favorable safety profile1 No DURAVYU-related SAEs Secondary: Reduction in treatment burden vs. 6 mos prior 89% 85% Secondary: Reduction in treatment burden vs. aflibercept 83% 79% Secondary: Supplement-free up to 6 months 65% 88% of eyes had 0 or only 1 supplemental injections 64% 83% of eyes had 0 or only 1 supplemental injections Secondary: Anatomical control vs. aflibercept +9.7um +5.2um

DURAVYU 2mg -0.3* Mean Change in BCVA vs Aflibercept DURAVYU 3mg -0.4* ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. +0.9* +1.0* +1.3* DURAVYU was Statistically Non-Inferior in Change in BCVA Compared to the Aflibercept Control (95% CI) MEAN CHANGE IN BCVA FROM BASELINE *Blended week 28 and week 32 change vs. baseline **Month 8 represents 6 months after DURAVYU injection CI, Confidence Interval PRELIMINARY DATA – PENDING FINAL ANALYSIS In the Pulsar trial, HD Eylea (16-week 8mg arm) change in BCVA vs. 2mg Eylea was -1.4 letters1 1 – AAO 2022 presentation, Paolo Lanzetta, on behalf of the PULSAR study investigators

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 95% Confidence Intervals Showed Statistical Non-Inferiority for Primary Endpoint with DURAVYU vs Aflibercept Control PRELIMINARY DATA – PENDING FINAL ANALYSIS BCVA units were ETDRS letters. BCVA, best-corrected visual acuity; CI, confidence interval; ETDRS, Early Treatment Diabetic Retinopathy Study; FDA, Food and Drug Administration; q16W, every 16 weeks. Mean Change in BCVA from Baseline Superiority Inferiority FDA LOWER BOUND FOR NI NO DIFFERENCE IN MEAN BCVA Mean -0.3 Mean -0.4 Predefined non-inferiority margin -4.5 DURAVYU 2 mg P = 0.00091 DURAVYU 3 mg P = 0.00019

DURAVYU Demonstrated a Favorable Safety Profile in the Phase 2 DAVIO 2 Clinical Trial1 ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. SAE, serious adverse event; AE, adverse event; IVT, intravitreal injection PRELIMINARY DATA CUT– PENDING FINAL ANALYSIS 1- As of November 7, 2023 data cut 2- as deemed by the investigator No reported DURAVYU-related ocular or systemic SAEs Four ocular SAEs reported in a study eye – none deemed DURAVYU related2 >97% of AEs reported were mild (Grade 1 or 2) and generally expected with IVT No insert migration into the anterior chamber No retinal occlusive vasculitis Low patient discontinuation rate of 4% up to week 32 No discontinuations were related to DURAVYU treatment

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Data as of April 25, 2024 1-Data are preliminary pending study completion and final report. SAE, serious adverse event Summary: DAVIO (Phase 1): 17 patients treated DAVIO 2 (Phase 2)1: 102 patients treated PAVIA (Phase 2)1: 51 patients treated 170 treated patients with a minimum of eleven months post DURAVYU injection with no DURAVYU-related ocular or systemic SAE’s DURAVYU Continues to Show a Favorable Safety Profile Across Multiple Clinical Trials

Clinically Meaningful Reduction in Treatment Burden Supports DURAVYU as a Maintenance Treatment For Wet AMD ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *Normalized PRELIMINARY DATA – PENDING FINAL ANALYSIS DURAVYU 2mg DURAVYU 3mg Mean number of injections week 8 through week 32 0.55 0.71 Mean number of injections 6 months prior to screening* 5.07 4.98 Reduction in treatment burden vs. 6 months prior (%) 89% 85%

DURAVYU Demonstrated a Meaningful Reduction in Treatment Burden vs. the Aflibercept Control Arm ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. PRELIMINARY DATA – PENDING FINAL ANALYSIS. DURAVYU 2mg DURAVYU 3mg Aflibercept 2mg q8W Mean number of injections week 8 through week 32 0.55 0.71 3.32 Reduction in treatment burden vs. aflibercept control (%) 83% 79% NA

DURAVYU Demonstrated Clinically Meaningful Supplement-Free Rates with ≥83% of Eyes Receiving 0-1 Anti-VEGF Supplemental Injections ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *PRELIMINARY DATA – PENDING FINAL ANALYSIS Number of Supplemental Injections Six Months After DURAVYU Insert DURAVYU 2mg (n = 50) 32 65.3% 11 22.4% 5 10.2% 1 2.0% 43 87.8% DURAVYU 3mg (n = 52) 32 61.5% 11 21.2% 3 5.8% 6 11.5% 43 82.7% 0 1 2 3+ # of Supplemental Injections
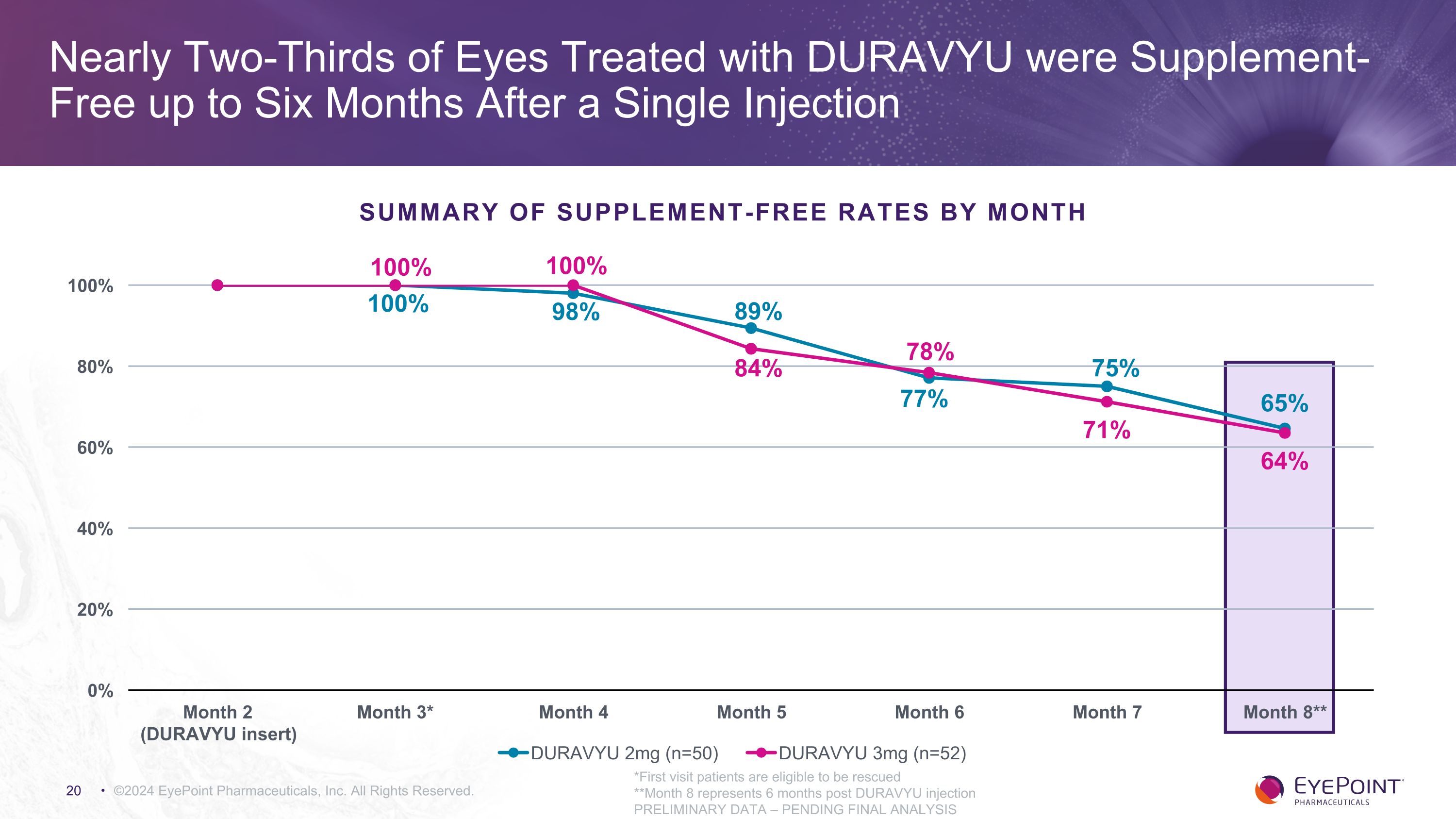
Nearly Two-Thirds of Eyes Treated with DURAVYU were Supplement-Free up to Six Months After a Single Injection ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *First visit patients are eligible to be rescued **Month 8 represents 6 months post DURAVYU injection PRELIMINARY DATA – PENDING FINAL ANALYSIS 100% Summary of Supplement-Free Rates by Month 100%
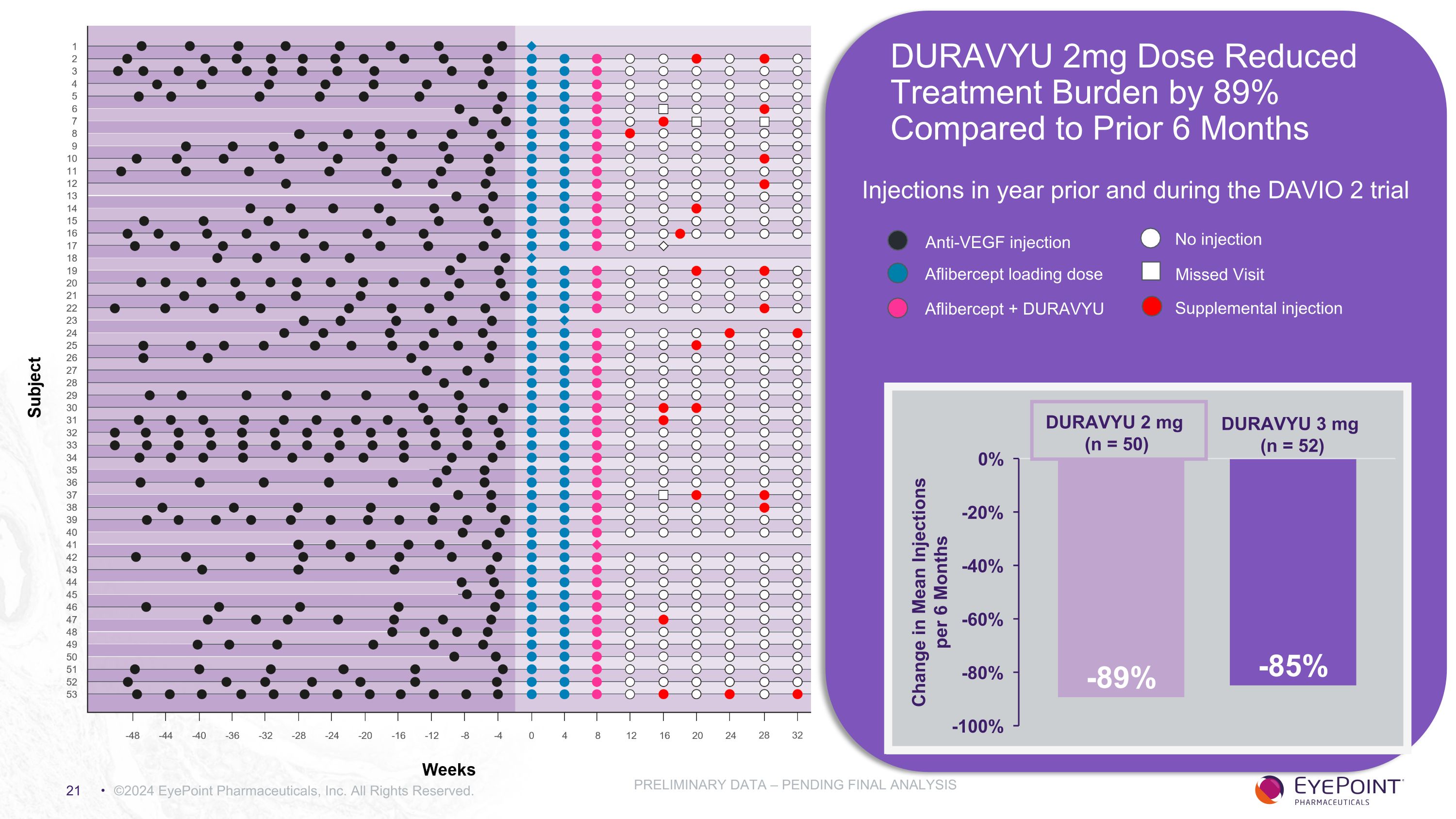
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. PRELIMINARY DATA – PENDING FINAL ANALYSIS DURAVYU 2mg Dose Reduced Treatment Burden by 89% Compared to Prior 6 Months Injections in year prior and during the DAVIO 2 trial DURAVYU 3 mg (n = 52) DURAVYU 2 mg (n = 50) Anti-VEGF injection Aflibercept loading dose Aflibercept + DURAVYU No injection Missed Visit Supplemental injection
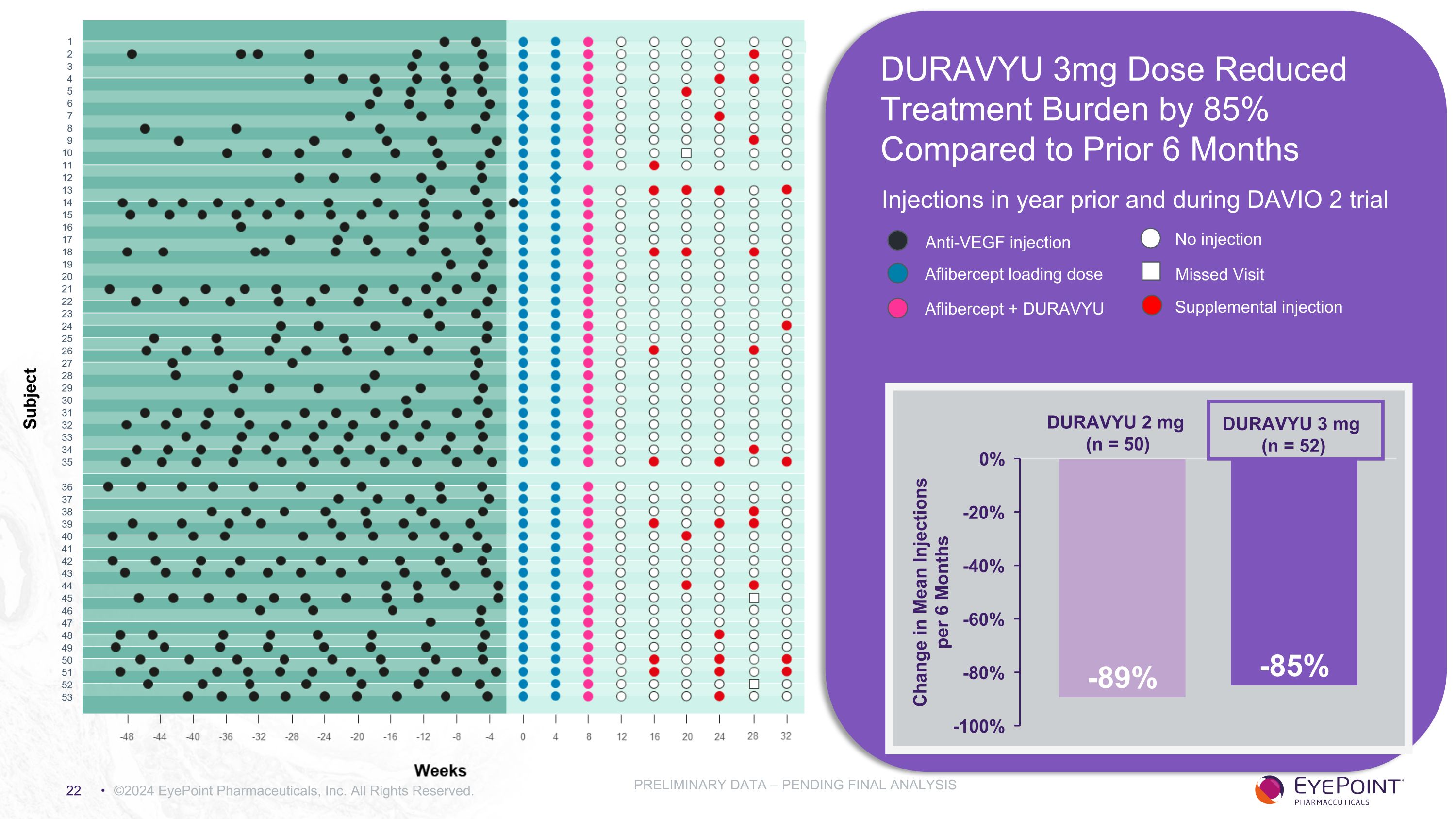
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. PRELIMINARY DATA – PENDING FINAL ANALYSIS Injections in year prior and during DAVIO 2 trial DURAVYU 3 mg (n = 52) DURAVYU 2 mg (n = 50) Anti-VEGF injection Aflibercept loading dose Aflibercept + DURAVYU No injection Missed Visit Supplemental injection DURAVYU 3mg Dose Reduced Treatment Burden by 85% Compared to Prior 6 Months
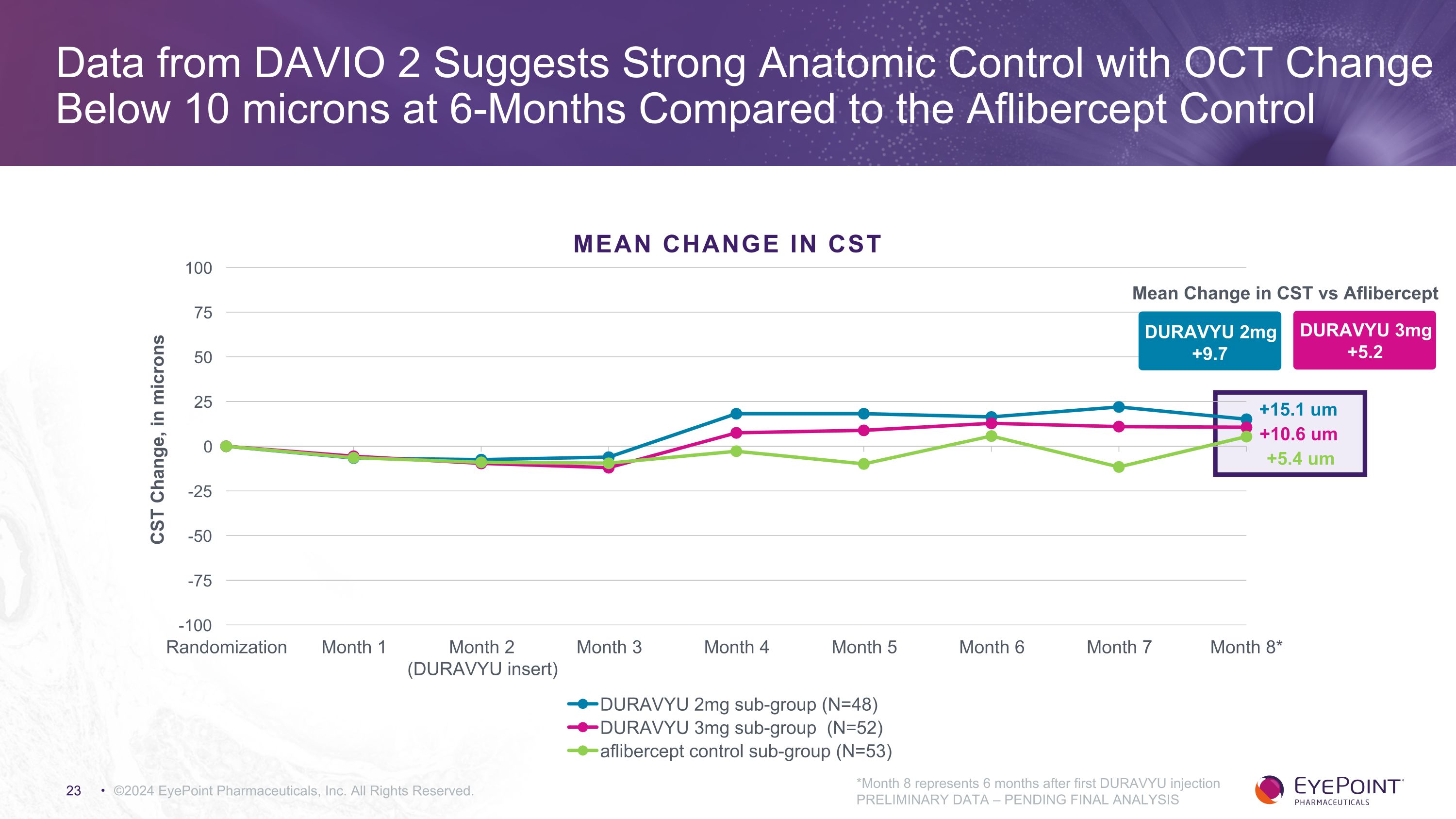
DURAVYU 2mg +9.7 Mean Change in CST vs Aflibercept DURAVYU 3mg +5.2 ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. +10.6 um +15.1 um +5.4 um *Month 8 represents 6 months after first DURAVYU injection PRELIMINARY DATA – PENDING FINAL ANALYSIS Data from DAVIO 2 Suggests Strong Anatomic Control with OCT Change Below 10 microns at 6-Months Compared to the Aflibercept Control MEAN CHANGE in CST

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Phase 2 DAVIO 2 Trial in Wet AMD��Sub-Group Analysis of Patients Anti-VEGF Supplement-Free Up to 6 Months

DURAVYU 2mg +0.6* Mean Change in BCVA vs Aflibercept DURAVYU 3mg +0.1* ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. +1.9* +2.4* +1.8* *Blended week 28 and week 32 change vs. baseline **Month 8 represents 6 months after DURAVYU injection PRELIMINARY DATA – PENDING FINAL ANALYSIS DURAVYU Demonstrated Numerical Superiority in Change in BCVA in Sub-Group Analysis of Patients Supplement-Free Up to 6-Months Sub-Group Analysis of Patients Supplement-Free Up to Six Months MEAN CHANGE IN BCVA FROM BASELINE

DURAVYU 2mg +7.0 Mean Change in CST vs Aflibercept DURAVYU 3mg +0.6 ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. +8.6 um +15.0 um +8.0 um *Month 8 represents 6 months after first DURAVYU injection PRELIMINARY DATA – PENDING FINAL ANALYSIS Strong Anatomic Control in Patients Supplement Free Up to 6-Months with OCT Change Below 10 microns Compared to the Aflibercept Control Sub-Group Analysis of Patients Supplement-Free Up to Six Months MEAN CHANGE in CST

s ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Phase 3 Pivotal Trials Design* NON-INFERIORITY VERSUS AN AFLIBERCEPT CONTROL *Pending final FDA review.

DURAVYU Non-Inferiority Phase 3 Clinical Trials Design in Wet AMD ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Design of the Phase 3 trials were informed by previous Type C meeting with FDA and positive DAVIO 2 data with additional considerations for potential FDA approval and product label. Positive EOP2 meeting with FDA completed in April 2024; waiting for final FDA review* Key trial design elements agreed upon with FDA: Two pivotal, non-inferiority trials vs. aflibercept control 12-month primary efficacy endpoint (blended) – basis of NDA submission DURAVYU re-dosing at six-month intervals – 4 total doses Masking strategy We remain on-track to initiate the LUGANO trial (US) in 2H 2024 with LUCIA trial (US/OUS) to follow. *Timing TBD based on extenuating circumstances with the interim FDA Division of Ophthalmology leadership required for sign-off. FDA, Food and Drug Administration; NDA, New Drug Application; OUS, outside the United States; EOP2, end of Phase 2

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU: vorolanib in Durasert E™ Phase 2 VERONA clinical trial IN Diabetic Macular Edema (DME)

Phase 2 VERONA Clinical Trial is a Randomized, Open-Label, Aflibercept Controlled Trial with a Single DURAVYU Injection DURAVYU dosing Visit Scheduled aflibercept injection Sham injection DURAVYU low dose (n=10) DURAVYU high dose (n=10) Aflibercept 2mg single injection (n=5) Supplemental Anti-VEGF injection based on prespecified criteria Potential 6-month treatment in previously treated DME patients Objectives: Evaluate the safety and efficacy of two doses of DURAVYU in the DME patient population Collect dose-ranging data to inform future clinical trials Primary endpoint: time to supplemental anti-VEGF injection up to week 24 Secondary endpoints: Change in BCVA vs. aflibercept control, stable anatomical outcome as measured by OCT, DRSS over time Primary endpoint -D28 to -D7 D1 W4 W8 W12 W16 W20 W24 ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Trial underway; topline data anticipated in Q1 2025

VERONA Primary Endpoint: Time to Supplemental Injection up to Week 24 – Supplement Criteria ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 1. vs. best on study measurement; 2. as measured by Spectral domain OCT (SD-OCT) BCVA, best corrected visual acuity; CST, central subfield thickness Starting at Week 4: Reduction in BCVA ≥10 letters due to DME1 Reduction in BCVA of 5-9 letters and >75 microns of new fluid at two consecutive visits1 Increase of ≥100 microns of new fluid vs. Baseline (Day 1)2 Investigator discretion Starting at Week 12: Lack of 10% reduction in CST compared to Baseline (Day 1)

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. EYP-2301: razuprotafib in Durasert E™ A sustained delivery tie-2 agonist for severe retinal diseases

EYP-2301: Razuprotafib in Durasert E™ is Being Developed as a Sustained Delivery Treatment for Serious Retinal Diseases ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 1. Heier et al. Retina, 2021;41:1-19. and Joussen et al. Eye 2021; 35:1305-1316.; 2. Hammes, et. Al – Diabetes.2011 Jan 1; 3. Shen et al. JCI, 2014; 124:4564; 4. Campochiaro et al. Ophthalmology, 2016; 123:1722-1730 Tie-2 activation combined with VEGF inhibition has the potential to enhance efficacy and extend durability1 of treatment In the retina, activated TIE-2 controls endothelial cell proliferation, barrier function and intercellular contacts, stabilizing vessels and the blood-retinal barrier2 Razuprotafib (f/k/a AKB-9778) delivered subcutaneously was previously studied demonstrating preclinical and clinical proof of concept in posterior segment disease 3,4 EYP-2301 targets vascular endothelial protein tyrosine phosphatase (VE-PTP) to promote TIE-2 activation and maintain vascular stability in the retina P P EYP-2301 Blood vessel lumen Intracellular space VE-PTP ANG2

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Cash runway through topline data in 2026 of pivotal Phase 3 clinical trials for DURAVYU in wet AMD Strong Balance Sheet $299M of cash and investments on March 31, 2023 No debt

Continued Execution And Well-Funded Through Key DURAVYU Milestones ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *EOP2, End of Phase 2; wet AMD, wet age-related macular degeneration; SAB, Scientific Advisory Board DURAVYU™ Corporate ✓ VERONA - DME Phase 2 Trial initiation Q1 2024 ✓ FDA conditional approval of DURAVYU proprietary name March 2024 ✓ EOP2 meeting with FDA for wet AMD Q2 2024 ✓ PAVIA topline data Q2 2024 DAVIO 2 12-month data Q2 2024 PAVIA 12-month data Q3 2024 First wet AMD Phase 3 trial (LUGANO) initiation 2H 2024 VERONA topline data Q1 2025 ✓ Appointed new Chief Medical Officer March 2024 ✓ Expanded SAB with world-renowned retina specialists April 2024 R&D Day June 2024

Investor Presentation May 2024 ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved.
v3.24.1.1.u2
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
EyePoint Pharmaceuticals (NASDAQ:EYPT)
Gráfico Histórico do Ativo
De Mai 2024 até Jun 2024

EyePoint Pharmaceuticals (NASDAQ:EYPT)
Gráfico Histórico do Ativo
De Jun 2023 até Jun 2024
