UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
December 2024
Commission File Number: 001-38723
Tiziana Life Sciences LTD
(Exact Name of Registrant as Specified in Its Charter)
9th Floor
107 Cheapside
London
EC2V 6DN
(Address of registrant’s principal executive
office)
Indicate by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
INFORMATION CONTAINED IN THIS REPORT ON FORM 6-K
On December 17, 2024, Tiziana Life Sciences LTD (the “Company”)
issued this 6K announcing, a significant milestone in its clinical development program for Alzheimer’s disease. The Company has
successfully dosed the first patient with moderate Alzheimer's disease using intranasal foralumab at Brigham and Women’s Hospital
in Boston, Massachusetts following on from their baseline PET scan., a copy of which is furnished as Exhibit 99.1
The Announcement is furnished herewith as Exhibit
99.1 to this Report on Form 6-K. The information in the attached Exhibits 99.1 is being furnished and shall not be deemed “filed”
for the purposes of Section 18 of the Securities Exchange Act of 1934, or otherwise subject to the liabilities of that Section, nor
shall it be deemed incorporated by reference in any filing made by the Company under the Securities Act of 1933, as amended, or the Securities
Exchange Act of 1934, except as otherwise set forth herein or as shall be expressly set forth by specific reference in such a filing.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
TIZIANA LIFE SCIENCES LTD |
| |
|
|
|
| Date: December 17, 2024 |
By: |
/s/ Keeren Shah |
| |
|
Name: |
Keeren Shah |
| |
|
Title: |
Chief Financial Officer |
EXHIBIT INDEX
3
Exhibit 99.1

Tiziana Life Sciences Announces First Patient
with Moderate Alzheimer’s Disease Dosed with Intranasal Foralumab
NEW YORK, December 17, 2024 – Tiziana Life Sciences, Ltd. (Nasdaq:
TLSA) (“Tiziana” or the “Company”), a biotechnology company developing breakthrough immunomodulation therapies
with its lead development candidate, intranasal foralumab, a fully human, anti-CD3 monoclonal antibody, today announced a significant
milestone in its clinical development program for Alzheimer’s disease. The Company has successfully dosed the first patient with
moderate Alzheimer’s disease using intranasal foralumab at Brigham and Women’s Hospital in Boston, Massachusetts following on from
their baseline PET scan.
Alzheimer’s disease represents an almost insurmountable global
health challenge, affecting millions worldwide with few treatment options available. Tiziana’s novel approach focuses on reducing
inflammation in the brain by addressing one of the major underlying inflammatory mechanisms believed to contribute to disease progression.
Foralumab delivered intranasally, offers a unique mechanism of action
by reducing brain microglial inflammation in patients with Alzheimer’s disease. This treatment strategy differs from beta amyloid
removal or tau protein reductions and relies on the stimulation of T regulatory cells. The activated regulatory T cells cross the blood
brain barrier where they reduce neuroinflammation of glial cells.
Dr. Howard Weiner, Principal Investigator, Chairman of Tiziana’s
Scientific Advisory Board and co-director of the Ann Romney Center for Neurologic Diseases at Brigham and Women’s Hospital, a founding
member of Mass General Brigham healthcare system, commented, “We are excited to initiate this study with Tiziana’s innovative intranasal
Foralumab in this first patient with moderate Alzheimer’s disease. There are no approved drugs to treat this stage of Alzheimer’s
disease. Targeting neuroinflammation represents a promising approach in the pursuit of disease-modifying therapies.”
Tiziana’s CEO, Ivor Elrifi, added, “Dosing the first patient
with intranasal Foralumab marks a significant milestone for Tiziana and underscores our commitment to advancing novel therapies for Alzheimer’s
disease. We look forward to the continued progress of this study and also plan a study of Foralumab in patients with mild Alzheimer’s
disease. Foralumab has the potential to make a meaningful difference for patients and their families.”
The study at Brigham and Women’s Hospital is part of Tiziana’s
broader development program for Foralumab, which includes other inflammatory and autoimmune indications. The company remains dedicated
to advancing scientific innovation to address unmet medical needs across diverse therapeutic areas.
In September 2024, the National Institutes of Health (NIH), National
Institute on Aging awarded a $4 Million grant to Dr. Howard Weiner as principal investigator at Brigham and Women’s Hospital to
be the key research site to study nasal anti-CD3 for the treatment of Alzheimer’s disease (AD). This significant grant is funding
a key research study over the next several years, advancing preclinical and ultimately, clinical studies of intranasal anti-CD3 as a potential
treatment for this devastating neurodegenerative condition.
About Foralumab
Foralumab, a fully human anti-CD3 monoclonal antibody, is a biological
drug candidate that has been shown to stimulate T regulatory cells when dosed intranasally. At present, 10 patients with Non-Active Secondary
Progressive Multiple Sclerosis (na-SPMS) have been dosed in an open-label intermediate sized Expanded Access (EA) Program with either
an improvement or stability of disease seen within 6 months in all patients. The FDA has recently allowed an additional 20 patients to
be enrolled in this EA program. In addition, intranasal foralumab is currently being studied in a Phase 2a, randomized, double-blind,
placebo-controlled, multicenter, dose-ranging trial in patients with non-active secondary progressive multiple sclerosis (NCT06292923).
Activated T cells play an important role in the inflammatory process.
Foralumab, the only fully human anti-CD3 monoclonal antibody (mAb) currently in clinical development, binds to the T cell receptor and
dampens inflammation by modulating T cell function, thereby suppressing effector features in multiple immune cell subsets. This effect
has been observed in patients with COVID and with multiple sclerosis, as well as in healthy normal subjects. The non-active SPMS intranasal
foralumab Phase 2 trial (NCT06292923) began screening patients in November of 2023. Immunomodulation by nasal anti-CD3 mAb represents
a novel avenue for treatment of neuroinflammatory and neurodegenerative human diseases.1,2
About Tiziana Life Sciences
Tiziana Life Sciences is a clinical-stage biopharmaceutical company
developing breakthrough therapies using transformational drug delivery technologies to enable alternative routes of immunotherapy. Tiziana’s
innovative nasal approach has the potential to provide an improvement in efficacy as well as safety and tolerability compared to intravenous
(IV) delivery. Tiziana’s lead candidate, intranasal foralumab, which is the only fully human anti-CD3 mAb currently in clinical
development, has demonstrated a favorable safety profile and clinical response in patients in studies to date. Tiziana’s technology
for alternative routes of immunotherapy has been patented with several applications pending and is expected to allow for broad pipeline
applications.
For more information about Tiziana Life Sciences and its innovative
pipeline of therapies, please visit www.tizianalifesciences.com.
| 1 | https://www.pnas.org/doi/10.1073/pnas.2220272120 |
| 2 | https://www.pnas.org/doi/10.1073/pnas.2309221120 |
Forward-Looking Statements
Certain statements made in this announcement are forward-looking statements.
These forward-looking statements are not historical facts but rather are based on the Company’s current expectations, estimates, and projections
about its industry, its beliefs, and assumptions. Words such as ‘anticipates,’ ‘expects,’ ‘intends,’ ‘plans,’ ‘believes,’ ’seeks,’ ‘estimates,’
and similar expressions are intended to identify forward-looking statements. These statements are not guarantees of future performance
and are subject to known and unknown risks, uncertainties, and other factors, some of which are beyond the Company’s control, are difficult
to predict, and could cause actual results to differ materially from those expressed or forecasted in the forward-looking statements.
The Company cautions security holders and prospective security holders not to place undue reliance on these forward-looking statements,
which reflect the view of the Company only as of the date of this announcement. Actual results may
differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the uncertainties
related to market conditions and other factors described more fully in the section entitled ‘Risk Factors’ in Tiziana’s
Annual Report on Form 20-F for the year ended December 31, 2023, and other periodic reports filed with the Securities and Exchange Commission.The
forward-looking statements made in this announcement relate only to events as of the date on which the statements are made. The Company
will not undertake any obligation to release publicly any revisions or updates to these forward-looking statements to reflect events,
circumstances, or unanticipated events occurring after the date of this announcement except as required by law or by any appropriate regulatory
authority.
For further inquiries:
Tiziana Life Sciences Ltd
Paul Spencer, Business Development, and Investor Relations
+44 (0) 207 495 2379
email: info@tizianalifesciences.com
Tiziana Life Sciences (NASDAQ:TLSA)
Gráfico Histórico do Ativo
De Nov 2024 até Dez 2024
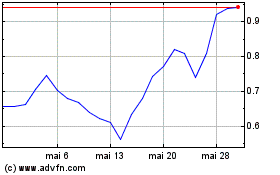
Tiziana Life Sciences (NASDAQ:TLSA)
Gráfico Histórico do Ativo
De Dez 2023 até Dez 2024